Enzyme engineering
Technical advances during the last decade have enabled researchers to modify at will the properties of many naturally occurring enzymes. These approaches have also facilitated the generation of stabilised enzymes with increased turnover numbers and altered substrate- and stereo-selectivities to be used in industrial processes. Up to now, the most impressive results in enzyme design have been obtained by directed evolution. In this two-step approach random mutagenesis is used to create large enzyme repertoires, from which optimised variants are then isolated using either selection or screening techniques. In contrast to directed evolution, the alternative approach of rational enzyme design requires a detailed knowledge of a specific enzyme structure and catalytic mechanism. Although occasionally successful, rational design approaches often fail, due to a limited understanding of the subtle interplay among amino acid side chains within an enzyme active site. Recent results suggest that this bottleneck toward the acquisition of tailored enzymes can be overcome by applying sophisticated computational methods.
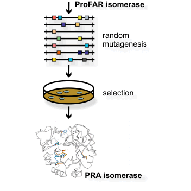
We have used directed evolution to establish the activity of phosphoriboslyanthranilate isomerase (TrpF) on the natural (βα)8-barrel scaffolds of ProFAR isomerase (HisA) and imidazoleglycerol phosphate synthase (HisF). Since only few mutations were necessary for the activity switch, our results suggest that TrpF, HisA and HisF have evolved from a common ancestor by a series of gene duplication and fusion events.
Moreover, we have used random mutagenesis and selection in vivo to increase the low catalytic activity of a thermostable dimeric anthranilate phosphoribosyl transferase at room temperature. The turnover number of the best mutant was increased 40-fold compared to the wild-type enzyme, due to an increased product release rate. Moreover, we have used rational design to generate a fully active monomeric variant of this enzyme by replacing hydrophobic residues at the protein interface with negatively charged ones.
-->