Absence of collagen IX seriously compromises growth cartilage structure and differentiation in mice
![]()
Skeletal development, growth, and repair mainly occur by endochondral ossification which comprises an orderly sequence of consecutive steps of proliferation and late differentiation of chondrocytes. After vascular invasion into hypertrophic cartilage, the tissue is remodelled into bone. At all stages, the process is under tight environmental control exerted by a combination of regulators, including nutritional supply and signalling through growth factors, hormones, and cell-matrix-interactions. The interaction of chondrocytes with their surrounding matrix via cell surface receptors is thought to play a key role in the regulation of survival, proliferation and maturation of cartilage cells. Therefore, genetic elimination of collagen IX, a stabilizing component of the periphery of thin cartilage fibrils, is expected to compromise extracellular matrix properties and, hence, the chondrocyte environment required for normal cartilage development and homeostasis. The current research was initiated to define the role of collagen IX for the cellular and fibrillar organization of the growth plate and its function during endochondral ossification.
Our data show that the growth plate morphology of Col9a1 -/- mice is markedly altered with changes being most prominent in late proliferative, pre-hypertrophic and hypertrophic and less obvious in resting and early proliferative zones. In the central region of newborn bones hypocellular areas with clearly reduced glycosaminoglycan content are observed. The columnar arrangement is profoundly disturbed and aberrant proliferation of chondrocytes in horizontal direction was detected.
In adult mice however, these alterations become attenuated and less prominent. Histomorphometric analyses reveal an irregular hypertrophic zone with a strongly decreased number of hypertrophic cells per area in the Col9a1-/- mice, especially at later time points. Narrowing of this zone seems to be caused by a significantly reduced proliferation rate in the proliferating zone of the growth plate. These morphological changes are associated with shortening and broadening of all long bones in newborn Col9a1 -/- mice.
Collagen IX stabilizes the macromolecular alloy of cartilage collagen fibrils therefore, when collagen IX is absent not only the cartilage fibrils themselves but also integration of other structural components within the cartilage matrix is compromised. In an earlier study we have shown that integration of matrilin-3 and COMP into a collagen IX-deficient matrix is transient and less stable. Because matrilins form complexes with proteoglycans, we suggest loss of matrilins due to the absence of collagen IX may contribute to the distorted distribution of proteoglycans. Latter are known to sequester growth factors which controlled delivery is responsible for normal proliferation and maturation of chondrocytes. Collagen IX therefore, plays a critical role for normal skeletal growth around birth and adolescent animals. However, we suggest that lack of collagen IX is compensated during growth since adult animals are similar in size to wild type mice.
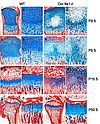
Histological analysis of skeletal defects in tibial growth plates
Weigert's hematoxylin/alcian blue/sirius red stained sections of wild type ( a-h ) and Col9a1-/- mice ( i-p ) tibiae reveal profoundly altered growth plate morphology at different postnatal ages (P0.5, P5.5, P15.5 and P50.5). The most prominent changes are: 1. Occurrence of a hypocellular area in the centre of epiphyseal cartilage extending into the hypertrophic zone at later stages; 2. Irregular alcian blue staining of the epiphyseal cartilage representing a disturbed glycosaminoglycan distribution; 3. Compromised formation of proliferation columns perpendicular rather than parallel to the long axis of the bone. All skeletal defects become attenuated with increasing age of knockout animals. Bars represent 250 µm.
Grants
This work was supported by the Deutsche Forschungsgemeinschaft:
grant GR1301/ 4-3 assigned to Susanne Grässel
Investigators
Prof. Dr. Susanne Grässel, Dr. Alfred Opolka, Mandy Vogel, Orthopaedic Surgery, University Hospital Regensburg
Collaboratros
Prof. Dr. Pter Bruckner, Dr. Rita Dreier, Institute for Physiological Chemistry & Pathobiochemistry, Univsersity Hospital Münster
Selected Publications
1. Budde B., Blumbach K., Ylästalo J, Zaucke F., Wagener R., Ehlen HWA, Ala-Kokko L., Paulsson M., Bruckner P., Grässel S.: Altered integration of matrilin –3 into cartilage extracellular matrix in the absence of collagen IX. Mol. Cell Biol. 25 (23): 10465-10478, 2005
,
2. Opolka A., Ratzinger S.,Schubert T.E.O., Spiegel H.-U., Grifka J., Bruckner P., Probst A., Grässel S.: Collagen IX is indispensable for timely maturation of cartilage during fracture repair in mice.: Matrix Biology, 26: 85-95, 2006
-->