Research
| We are analyzing the control of the cell cycle at genetic, cellular and biochemical levels and are interested in the integration of the cell cycle with developmental processes. We are using the whole spectrum of the model organism Drosophila and cell culture systems. | |
| Ongoing projects and interests | |
 | Regulation of the Anaphase-Promoting-Complex/Cyclosome (APC/C) and its inhibitor Rca1 |
 | Protein destruction mediated by the Rca1-SCF-complex |
 | Regulation of "special" cell cycle, e.g. endoreduplication cycles |
 | The fastest eukaryotic cell cycle on earth How is it controlled? |
| Methods being used in the lab | |
| Regulation of the Anaphase-Promoting-Complex/Cyclosome (APC/C) and its inhibitor Rca1 | |
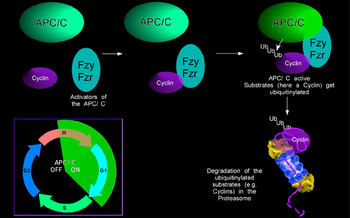
| The APC/C is a multisubunit ubiquitin-Ligase that is used to mark proteins for destruction during a cell cycle. The irreversible destruction of proteins is a common theme in cell cycle regulation. It ensures that the cell cycle goes in one direction and helps to reinforce transition steps. The attachment of ubiquitin to target proteins marks them for deletion in the proteasome. For an orderly cell cycle, the destruction of important cell cycle regulators has to be tightly controlled during the cycle. The APC/C ubiquitin ligase is normally active during mitosis and G1 where it is responsible for the degradation of many cell cycle proteins, like cyclins, securing and many others. However, cyclins are required for entry into mitosis and APC/C activity has to be restrained during S and G2 phase to allow cyclin accumulation. | |
| We have identified the gene rca1 as an essential inhibitor of the APC/C. In the absence of Rca1 function, cells fail to divide because cyclins, that are required for entry into mitosis are untimely degraded before mitosis by the APC/C. Thus, Rca1 is required to inhibit the APC/C before mitosis. Rca1 shares limited sequence homology with the APC/C inhibitors of the vertebrate Emi-family. For these proteins, it was proposed that they work by inhibiting the APC/C as a pseudosubstrate inhibitor. We would like to establish if Rca1 works in a similar manner and how APC/C inhibition is achieved. In addition, Rca1 itself has to be regulated during the cell cycle to relieve APC/C inhibition when the ubiquitin ligase should be active. We could observe that Rca1 is itself degraded during G1 and now would like to understand how the degradation is achieved and regulated. Besides its role during the cell cycle, the APC/C has been reported to be active and required in cells that have exited the cell cycle. By using APC/C sensors and APC/C mutations or RNAi knockdown procedures we would like to see if other, non cell-cycle functions of the APC/C are required. | |
| Protein destruction mediated by the Rca1-SCF-complex | |
| Another class of ubiquitin ligases that play important roles during the cell cycle is the SCF-complex which is composed of several proteins, including Skp1, Cul1, an F-box-containing protein and a protein that has ubiquitin-ligase activity. SCF complexes are evolutionarily conserved among eukaryotes; they are found in organisms from yeast to humans. The F-box proteins contain at least two domains: an F-box responsible for binding to Skp1 and another protein-protein interaction domain which is believed to interact with the substrate protein. | |
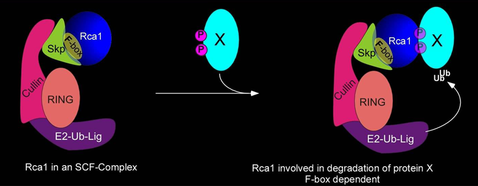 | |
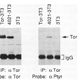
| The above-mentioned Rca1 protein contains the F-box motif and we could show that Rca1 interacts with Skp and cullin proteins. The F-box motif is not required for APC/C inhibition during the G2-phase, but Rca1 lacking the F-box is unable to fully complement rca1 function. Cells that only have Rca lacking the F-box fail to proliefate normally. We are interested what the function such Rca1 containing SCF-complexes have and what proteins might be targeted for degradation by the Rca1-SCF-complex. Both genetic and biochemical approaches are currently being used to identify these novel function of Rca1 | |
Regulation of "special" cell cycles types | |
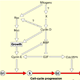
| The most common cell cycle type consists of 4 Phases: Mitosis when cell division takes place is usually followed by a so called “gap”-Phase, G1 before DNA-replication is initiated during S-phase that is followed by another gap-Phase, G2. | |
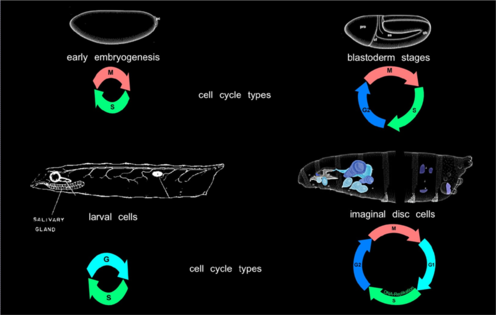 | |
Regulation of endoreduplication cycles | |
| The precise duplication of the genome is crucial for the survival of any organism. In multicellular organisms genome instability potentially gives rise to cancer and thus compromises the life of the whole organism. To maintain the integrity of the genome, DNA replication and mitosis must be coordinated during cell division cycles so that DNA replication occurs only once per cycle and mitosis only after complete duplication of the genome. To avoid re-replication events, a network of proteins ensures that cells acquire the license for DNA replication only in a specific phase of the cell cycle While re-replication without an intervening mitosis is strictly prevented during cell division cycles, this process is enforced during endoreplication cycles. This cell cycle variant is prominently found during plant and invertebrate development, but some mammalian cell types, like megakaryocytes or trophoblast giant cells, are also known to undergo endoreplication cycles. Endoreplicating cells undergo repeated rounds of DNA synthesis that are separated by distinct Gap phases, but never enter mitosis.
| |
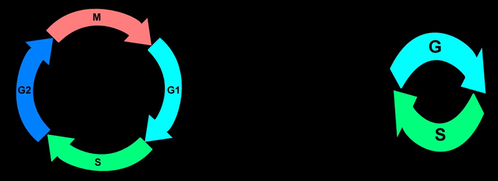 | |
| Despite the absence of mitosis, the Anaphase-Promoting Complex is required for endoreduplication cycles. However, the degradation of mitotic substrates like cyclins is of no importance here. Instead, APC/C is required for the degradation of an inhibitor, geminin that regulates the assembly of DNA-replication origns. When the APC/C is inhibited, Geminin accumulates and prevents reassembly of DNA-replication origins. Repeated rounds of S- and Gap phases are regulated by distinct pathways thar result in oscialltions of cell cycle regulators. At the heart of the system is Cyclin E, an unstable protein that requires transcriptional input from the transcription factor E2F. Once E2F can accumulate, it triggers DNA-replication and this in turn results in E2F degradation, hence reduced CycE. The APC/C is inhibited by CycE and only after a drop in CycE levels, APC/C is able to degrade geminin relieving the inhibition in DNA-Origins assembly | |
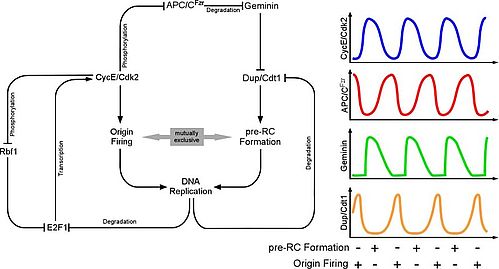 | |
| Regulation of early embryonic cell cycles in Drosophila | |
| The first divisions after fertilization are nuclear divisions that occur in a common cytoplasm. These cycles occur in an almost synchronous manner, consist of S- and M-Phase only are the fastest cell cycle known for eucaryotes. | |
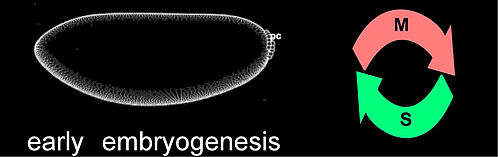 | |
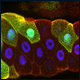
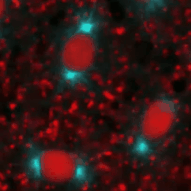
| The speed and synchrony as well as the relative big size of the embryo make this an ideal model to follow cell cycle events in real time. This is possible through the use of fluorescently marked proteins (see this years Nobel Prize in Chemisty) that are attached to proteins that bind to DNA (in Cyan) or Tubulin (red). In the movie on the right, three cycles from a part of an early Drosophila embryo are shown. The earliest cycles during embryogenesis are not easily explained by standard cell cycle models. Usually, a cell cycle is characterized by oscillations in cell cycle components or their activity. For example, Cdk1, a kinase that triggers mitosis, is only active shortly before mitosis when it promotes mitosis and needs to be inactivated when cells exit mitosis. However, in the early nuclear cycles, no fluctuations in Cdk1 activity can be observed. It remains to be investigated how these cycles are coordinated. | |
Team
 | Prof. Dr. rer. nat. Frank Sprenger Gebäude Westliche Naturwissenschaften D3_2.323 |
 | Arlett Hirsch Gebäude Westliche Naturwissenschaften D3_2.311 |
| Ph.D. Students | |
 | Manuel Saller |
 | Sebastian Sigl |
| former Ph.D. Students | |
 | Matthias Kies |
 | Thomas Rössler |
 | Jan Polz |
| Lab Manager | |
 | Christiane Sprenger Technische Assistentin Gebäude Westliche Naturwissenschaften D3_2.321 Tel. 0941 943-3168, Labor 3169 christiane.sprenger@ur.de |
| Frank Sprenger Curriculum Vitae | |
| Education | |
| 1980-1984 | Studies of Chemistry and Biochemistry, University Tübingen |
| 1984-1985 | Student in the Department of Molecular, Cellular and Developmental Biology, University of Colorado, Boulder, USA |
| 1985-1986 | Biochemistry student, University Tübingen |
| 1987 | Diploma thesis, Max-Planck-Institut for Developmental Biology, Tübingen with C. Nüsslein-Volhard |
| 1988-1991 | Ph.D. thesis at the Max-Planck-Institut for Developmental Biology, Tübingen with C. Nüsslein-Volhard |
| Degrees and promotions | |
| 1980 | Abitur |
| 1987 | Diploma |
| 1991 | Ph.D. |
| 2001 | Venia legendi |
| 2002 | Oberassistent |
| 2004 | Hochschuldozent |
| 2007 | Professor |
| Positions | |
| 1991 | Postdoc at the Max-Planck-Institut for Developmental Biology, Tübingen with C. Nüsslein-Volhard |
| 1992-1996 | Postdoc at the University of California, San Francisco, Department of Biochemistry and Biophysics with P. O'Farrell |
| 1997-2007 | Group leader at the University of Cologne, Institute for Genetics |
| 2008 | Senior research assistent, University of Zürich with C. Lehner |
| 2009- | Professor for Genetics, University of Regensburg |
| Honors and Stipends | |
| 1988 | Postgraduate Stipend, Boehringer Ingelheim Fonds |
| 1991 | Postdoc Fellowship European Molecular Biology Organization |
| 1992 | Otto Hahn Medal of the Max-Planck Society |
| 1992 | Postdoc Fellowship, Human Frontiers in Science Organization |
| 1996 | Independent groupleader position within SFB 243 |
Publications
 | Ayeni J, Varadarajan R, Mukherjee O, Stuart DT, Sprenger F, Campbell SD (2014) Dual phosphorylation of cdk1 coordinates cell proliferation with key developmental processes in Drosophila. Genetics, 196, 197-210 |
 | Lidsky PV, Sprenger F, Lehner CF. (2013) Distinct modes of centromere protein dynamics during cell cycle progression in Drosophila S2R+ cells. Journal of Cell Science, 126, 4782-93 |
 | Pellegrino, Mark W., Gasser, Robin B., Sprenger, Frank, Stetak, Attila, Hajnal, Alex (2009), The conserved zinc ï¬nger protein vab-23 is an essential regulator of epidermal morphogenesis in C. elegans. Developmental Biology, 336, 84-93 |
 | Zielke, N., Querings, S., Rottig, C., Lehner, C., and Sprenger, F. (2008). The anaphase-promoting complex/cyclosome (APC/C) is required for rereplication control in endoreplication cycles. Genes & Development 22, 1690-1703. |
 | Ramachandran, V, Matzkies, M, Dienemann, A and Sprenger, F. (2006). Cyclin A degradation employs preferentially used lysines and a cyclin box function other than Cdk1 binding. Cell Cycle, 6, 171-181 |
 | Zielke, N, Querings, S, Grosskortenahaus, R, Reis, T and Sprenger, F. (2006). Molecular dissection of the APC/C inhibitor Rca1 reveals a novel F-box dependent function. EMBO Rep, 7, 1266-1272 |
 | Dienemann, A and Sprenger, F (2004). Requirements of Cyclin A for mitosis are independent of its subcellular localization. Current Biology., 14, 1117-1123
|
 | Grosskortenhaus, R. and Sprenger, F. (2002). Rca1 inhibits APC-Cdh1(Fzr) and is required to prevent cyclin degradation in G2. Developmental Cell, 2, 29-40 |
 | Kaspar, M, Dienemann, A, Schulze, C and Sprenger, F. (2001). Mitotic degradation of Cyclin A is mediated by mediated by multiple and novel destruction signals. Current Biologie. 11(9), 685-690 |
 | Foley, E., and Sprenger, F. (2001). Rux is involved in exit from mitosis in Drosophila. Current Biologie 11,151-160 |
 | Foley, E., and Sprenger, F., (2000) Cyclins: Growing pains for Drosophila. Curr Biologie, 10, R665-R667 |
 | Niessing, D., Driever, W., Sprenger, F., Taubert, H., Jackle, H., and Rivera-Pomar, R.(2000), Homeodomain position 54 specifies transcriptional versus translational control by Bicoid. Molecular Cell, 5, 395-401 |
 | Foley, E, O'Farrell, P.H., Sprenger, F. (1999). Rux is a cyclin-dependent kinase inhibitor (CKI) specific for mitotic cyclin Cdk complexes. Current Biologie 9:1392-1402. |
 | Su, T. T., Sprenger, F., DiGregorio, P. J., Campbell, S. D., and O'Farrell, P. H. (1998). Exit from mitosis in drosophila syncytial embryos requires proteolysis and cyclin degradation, and is associated with localized dephosphorylation. Genes & Development 12, 1495-503. |
 | Sprenger, F., Yakubovitch, N., and O'Farrell, P. H. (1997). S-phase function of Drosophila cyclin A and its downregulation in G1 phase. Current Biology 7, 488-499. |
 | Furriols, M., Sprenger, F., and Casanova, J. (1996). Variation in the number of activated torso receptors correlates with differential gene expression. Development 122, 2313-2317. |
 | Baek, K. H., Fabian, J. R., Sprenger, F., Morrison, D. K., and Ambrosio, L. (1996). The activity of D-raf in torso signal transduction is altered by serine substitution, N-terminal deletion, and membrane targeting. Developmental Biologie 175, 191-204. |
 | Campbell, S. D., Sprenger, F., Edgar, B. A., and O'Farrell, P. H. (1995). Drosophila Wee1 kinase rescues fission yeast from mitotic catastrophe and phosphorylates Drosophila Cdc2 in vitro. Mol Biol Cell 6, 1333-1347. |
 | Edgar, B. A., Sprenger, F., Duronio, R. J., Leopold, P., and O'Farrell, P. H. (1994). Distinct molecular mechanism regulate cell cycle timing at successive stages of Drosophila embryogenesis. Genes & Development 8, 440-452. |
 | Sprenger, F., Trosclair, M. M., and Morrison, D. K . (1993). Biochemical analysis of torso and D-raf during Drosophila embryogenesis: implications for terminal signal transduction. Mol Cell Biol 13, 1163-1172. |
 | Sprenger, F., and Nusslein-Volhard, C. (1992). Torso receptor activity is regulated by a diffusible ligand produced at the extracellular terminal regions of the Drosophila egg. Cell 71, 987-1001. |
 | Dickson, B.,Sprenger, F., Morrison, D., and Hafen, E. (1992). Raf functions downstream of Ras1 in the Sevenless signal transduction pathway. Nature 360, 600-603. |
 | Dickson, B., Sprenger, F., and Hafen, E. (1992). Prepattern in the developing Drosophila eye revealed by an activated torso--sevenless chimeric receptor. Genes Dev 6, 2327-2339. |
 | Sprenger, F., Stevens, L. M., and Nusslein-Volhard, C. (1989). The Drosophila gene torso encodes a putative receptor tyrosine kinase. Nature 338, 478-483. |
| Book Chapters | |
| Sprenger, F., and Nüsslein-Volhard, C. (1993). The terminal system of axis determination in the Drosophila embryo. In:The Development of Drosophila melanogaster, M. Akam and A. Martinez-Arias, eds. (Plainview, New York: Cold Spring Harbor Laboratory Press), pp. 365-386. | |
| Klymkowsky, W. M., Christian, E., Ham, R. G., Plummer, D. J., and Sprenger, F. (1987). Giant axonal neuropathy mutations, intermediate filaments and cellular metabolism. In: Intrinsic determinants of neuronal form and function, R. Lasek and M. Black, eds. (New York: Alan Liss,), pp. 441-459. |
Teaching
Lehrveranstaltungen WS 2017/18 (LSF-Liste)
Lehrveranstaltungen SS 2018 (LSF-Liste)
Teaching Sommersemester
54119 Vorlesung Genetik
54120 Übung zur Vorlesung Genetik
54123 Grundkurs Genetik
54413 Seminar über laufende wissenschaftliche Arbeiten der Genetik
54415 Seminar zum Laborpraktikum: Regulation der Zellteilung in Drosophila
54422 Forschungspraktikum Genetik
54425 Laborpraktikum Genetik: Regulation der Zellteilung in Drosophila
54427 Journal Club: Cell Cycle Control Mechanisms in Eukaryotes
Teaching Wintersemester
54123 Wahlpflichtpraktikum Genetik
54402 Vorlesung: Cell Cycle Control
54413 Seminar über laufende wissenschaftliche Arbeiten der Genetik
54415 Seminar zum Laborpraktikum:: Regulation der Zellteilung in Drosophila
54417 Projektpraktikum: Molekulargenetik
54422 Forschungspraktikum Genetik
54425 Laborpraktikum Genetik: Regulation der Zellteilung in Drosophila
54427 Journal Club: Cell Cycle Control Mechanisms in Eukaryotes
54428 Workshop on digital image processing using ImageJ
Contact
| Prof. Dr. Frank Sprenger | ||||
| google map position |  | |||
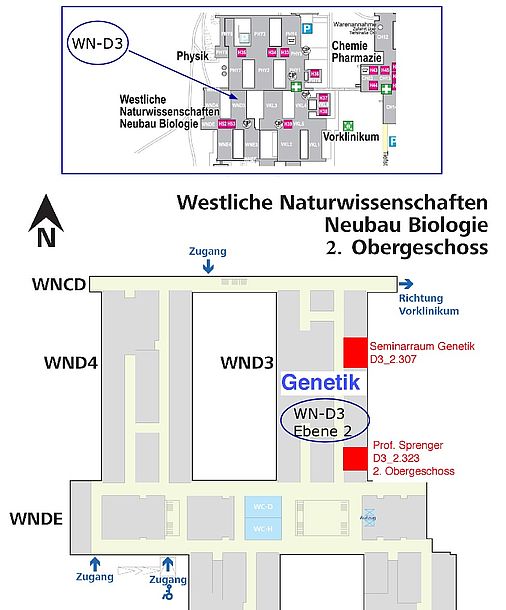
| Lageplan Neubau Biologie (Westliche Naturwissenschaften)
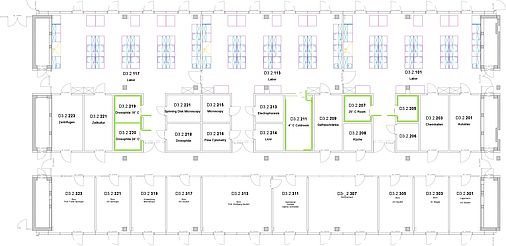
Lageplan Räume Genetik
How to get there The University has a good "How to get there" page:
| ||||