Startseite Physikalische Chemie - Prof. Dr. Hartmut Yersin
Welcome to the Homepage of Hartmut Yersin
Latest Publications
R.Czerwieniec, M.J. Leitl, H.H.H. Homeier, H. Yersin*
Thermally Cu(I) Complexes - Thermally activated delayed fluorescence. Photophysical approach and material design
Review: Coord. Chem. Rev. 2016, 325, 2-28
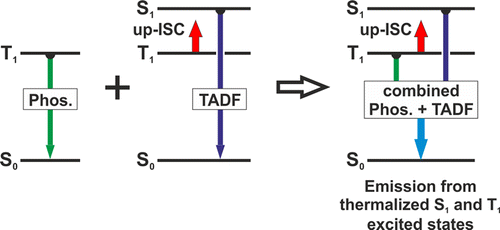
Cu(I) complexes often show electronic transitions of distinct metal-to-ligand charge transfer (MLCT) character. This can lead to small energy separations E(S1-T1) between the lowest singlet S1 and triplet T1 state. Hence, thermally activated delayed fluorescence (TADF) and, if applied to electroluminescent devices, singlet harvesting can become highly effective. In this contribution, we introduce the TADF mechanism and identify crucial parameters that are necessary to optimize materials´ properties, in particular, with respect to short emission decay times and high quantum yields at ambient temperature. In different case studies, we present a photophysical background for a deeper understanding of the materials properties.
T. Hofbeck, Y. C. Lam, M. Kalbáč, S. Záliš, A. Vlček* Jr., H. Yersin*
Thermally Tunable Dual Emission of the d8-d8 Dimer
[Pt2(µ-P2O5(BF2)2)4]4–
Inorg. Chem. 2016, 55, 2441
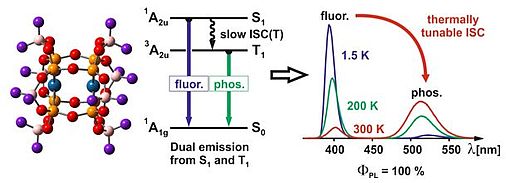
High-resolution fluorescence, phosphorescence, as well as related excitation spectra, and, in particular, the emission decay behaviour of solid [Bu4N][Pt2(µ-P2O5(BF2)2)4], abbreviated Pt(pop-BF2), have been investigated over a wide temperature range, 1.3 – 310 K. We focus on the lowest excited states that result from dσ*pσ (5dz2-6pz) excitations, i.e., the singlet state S1 (of 1A2u symmetry in D4h) and the lowest triplet T1, which splits into spin-orbit substates A1u(3A2u) and Eu(3A2u). After optical excitation, an unusually slow intersystem crossing (ISC) is observed. As a consequence, the compound shows efficient dual emission, consisting of blue fluorescence and green phosphorescence with an overall emission quantum yield of ≈100% over the investigated temperature range. Our investigation sheds light on this extraordinary dual emission behaviour, which is unique for a heavy-atom transition metal compound.
M. J. Leitl, D. M. Zink, A. Schinabeck, T. Baumann, D. Volz*, H. Yersin*
Copper(I) Complexes for Thermally Activated Delayed Fluorescence: From Photophysical to Device Properties
Review: Top. Curr. Chem. 2016, 374,1
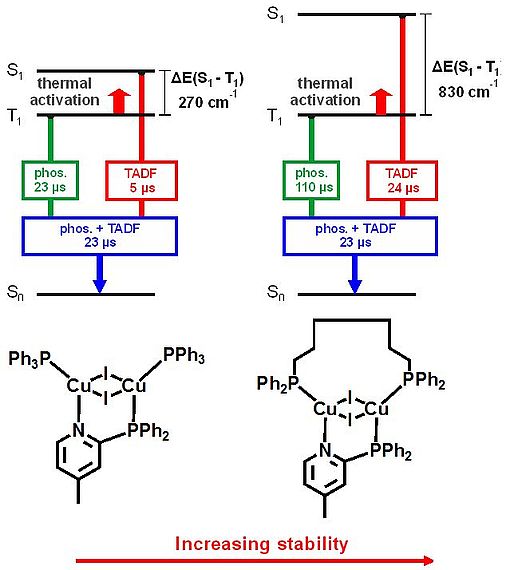
Molecules that exhibit thermally activated delayed fluorescence (TADF) represent a very promising emitter class for application in electroluminescent devices since all electrically generated excitons can be transferred into light according to the singlet harvesting mechanism. Cu(I) compounds are an important class of TADF emitters. In this contribution, we want to give a deeper insight into the photophysical properties of this material class and demonstrate how the emission properties depend on molecular and host rigidity. Moreover, we show that with molecular optimization a significant improvement of selected emission properties can be achieved. From the discussed materials, we select one specific dinuclear complex, for which the two Cu(I) centers are four-fold bridged to fabricate an organic light emitting diode (OLED). This device shows the highest efficiency (of 23 % external quantum efficiency) reported so far for OLEDs based on Cu(I) emitters.
T. Hofbeck, U. Monkowius, H. Yersin*
Highly Efficient Luminescence of Cu(I) Compounds: Thermally Activated Delayed Fluorescence with Short-Lived Phosphorescence
J. Am. Chem. Soc. 2015, 137, 399.
Luminescent materials showing thermally activated delayed fluorescence (TADF) have gained high attractiveness as emitters in organic light emitting diodes (OLEDs) and other photonic applications. Nevertheless, even utilization of TADF can be further improved, introducing a novel concept. This is demonstrated by a new class of brightly luminescent low-cost Cu(I) compounds, for which the emission stems from both the lowest excited triplet T1 and singlet S1 state. At T = 300 K, these materials exhibit quantum yields of more than ΦPL = 90 % at short emission decay times. About 80 % of the emission intensity stems from the singlet due to TADF, but importantly, an additional 20 % is contributed by the lower lying triplet state according to effective spin–orbit coupling (SOC). SOC induces also a relatively large zero-field splitting of the triplet being unusual for Cu(I) complexes. Thus, the overall emission decay time is distinctly reduced. Combined use of both decay paths opens novel photonic applications, in particular, for OLEDs.
M. J. Leitl, V. A. Krylova, P. I. Djurovich, M. E. Thompson*, H. Yersin*
Phosphorescence versus Thermally Activated Delayed Fluorescence. Controlling Singlet–Triplet Splitting in Brightly Emitting and Sublimable Cu(I) Compounds
J. Am. Chem. Soc. 2014, 136, 16032.
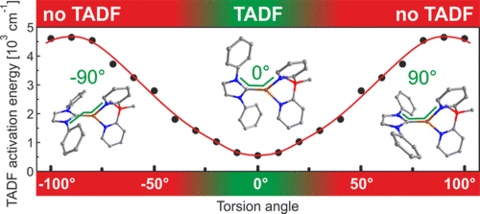
Photophysical properties of two highly emissive three-coordinate Cu(I) complexes, (IPr)Cu(py2-BMe2) (1) and (Bzl-3,5Me)Cu(py2-BMe2) (2), with two different N-heterocyclic (NHC) ligands were investigated in detail (IPr = 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene; Bzl-3,5Me = 1,3-bis(3,5-dimethylphenyl)-1H-benzo[d]imidazol-2-ylidene; py2-BMe2 = di(2-pyridyl)dimethylborate). The compounds exhibit remarkably high emission quantum yields of more than 70 % in the powder phase. Despite similar chemical structures of both complexes, only compound 1 exhibits thermally activated delayed blue fluorescence (TADF), whereas compound 2 shows a pure, yellow phosphorescence. This behavior is related to the torsion angles between the two ligands. Changing this angle has a huge impact on the energy splitting between the first excited singlet state S1 and triplet state T1 and therefore on the TADF properties. In addition, it was found that, in both compounds, spin–orbit coupling (SOC) is particularly effective compared to other Cu(I) complexes. This is reflected in short emission decay times of the triplet states of only 34 μs (1) and 21 μs (2), respectively, as well as in the zero-field splittings of the triplet states amounting to 4 cm–1 (0.5 meV) for 1 and 5 cm–1 (0.6 meV) for 2. Accordingly, at ambient temperature, compound 1 exhibits two radiative decay paths which are thermally equilibrated: one via the S1 state as TADF path (62 %) and one via the T1 state as phosphorescence path (38 %). Thus, if this material is applied in an organic light-emitting diode, the generated excitons are harvested mainly in the singlet state, but to a significant portion also in the triplet state. This novel mechanism based on two separate radiative decay paths reduces the overall emission decay time distinctly.
A. M. Prokhorov*, T. Hofbeck, R. Czerwieniec, A. F. Suleymanova, D. Kozhevnikov, H. Yersin*
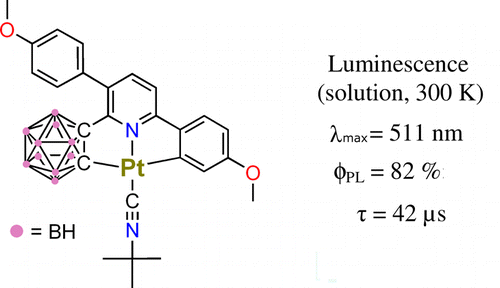
Brightly Luminescent Pt(II) Pincer Complexes with a Sterically Demanding Carboranyl-Phenylpyridine Ligand: A New Material Class for Diverse Optoelectronic Applications
J. Am. Chem. Soc. 2014, 136, 9637.
A series of three Pt(II) complexes with a doubly cyclometalating terdentate ligand L1, L1H2 = 3,6-bis(p-anizolyl)-2-carboranyl-pyridine, and diethyl sulfide (1), triphenylphosphine (2), and t-butylisonitrile (3) as ancillary ligands were synthesized. X-ray diffraction studies of 1 and 2show a coordination of the L1 ligand in a C–N–C mode in which the bulky and rigid o-carborane fragment is cyclometalated via a C atom. Importantly, no close intermolecular Pt–Pt contacts occur with this ligand type. The new Pt(II) pincer complexes display very high luminescence quantum yields at decay times of several tens of μs even in solution under ambient conditions. On the basis of the low-temperature (T = 1.3 K) emission decay behavior, the emission is assigned to a ligand centered triplet excited state 3LC with small 1,3MLCT admixtures. Because the phosphorescence is effectively quenched by molecular oxygen, optical sensors operating in a wide range of oxygen pressure can be developed. Owing to the very high luminescence quantum yields, the new materials might also become attractive as emitter materials for diverse optoelectronic applications.
T. Niehaus, T. Hofbeck, H. Yersin
Charge-transfer excited states in phosphorescent organo-transition metal compounds: a difficult case for time dependent density functional theory?
RSC Adv., 2015,5, 63318.
Light emitting organo-transition metal complexes have found widespread use in the past. The computational modelling of such compounds is often based on time-dependent density functional theory (TDDFT), which enjoys popularity due to its numerical efficiency and simple black-box character. It is well known, however, that TDDFT notoriously underestimates energies of charge-transfer excited states which are prominent in phosphorescent metal–organic compounds. In this study, we investigate whether TDDFT is providing a reliable description of the electronic properties in these systems. To this end, we compute 0–0 triplet state energies for a series of 17 pseudo-square planar platinum(II) and pseudo-octahedral iridium(III) complexes that are known to feature quite different localization characteristics ranging from ligand-centered (LC) to metal-to-ligand charge transfer (MLCT) transitions. The calculations are performed with conventional semi-local and hybrid functionals as well as with optimally tuned range-separated functionals that were recently shown to overcome the charge transfer problem in TDDFT. We compare our results against low temperature experimental data and propose a criterion to classify excited states based on wave function localization. In addition, singlet absorption energies and singlet–triplet splittings are evaluated for a subset of the compounds and are also validated against experimental data. Our results indicate that for the investigated complexes charge-transfer is much less pronounced than previously believed.
T. Gneuß, M. J. Leitl, L. H. Finger, N. Rau, H. Yersin*, J. Sundermeyer*
A New Class of Luminescent Cu(I) Complexes with Tripodal Ligands - TADF Emitters for the Yellow to Red Color Range
Dalton Trans. 2015, 44, 8506.
A new class of emissive and neutral Cu(I) compounds with tripodal ligands is presented. The complexes were characterized chemically, computationally, and photophysically. Under ambient conditions, the powders of the compounds exhibit yellow to red emission with quantum yields ranging from about 5 % to 35 %. The emission represents a thermally activated delayed fluorescence (TADF) combined with a short-lived phosphorescence which represents a rare situation and is a consequence of high spin–orbit coupling (SOC). In the series of the investigated compounds the non-radiative rates increase with decreasing emission energy according to the energy gap law while the radiative rate is almost constant. Furthermore, a well-fit linear dependence between the experimental emission energies and the transition energies calculated by DFT and TD-DFT methods could be established, thus supporting the applicability of these computational methods also to Cu(I) complexes.
R. Czerwieniec, H. Yersin*
Diversity of Copper(I) Complexes Showing Thermally Activated Delayed Fluorescence: Basic Photophysical Analysis
Inorg. Chem. 2015, 54, 4322.
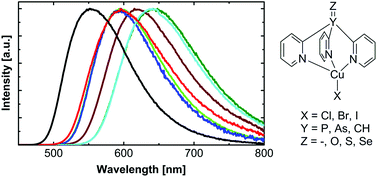
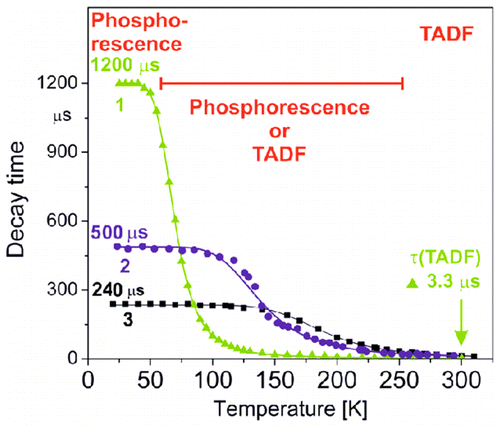
A comparison of three copper(I) compounds [1, Cu(dppb)(pz2Bph2); 2, Cu(pop)(pz2Bph2); 3, Cu(dmp)(phanephos)+] that show pronounced thermally activated delayed fluorescence (TADF) at ambient temperature demonstrates a wide diversity of emission behavior. In this study, we focus on compound 1. A computational density functional theory (DFT)/time-dependent DFT approach allows us to predict detailed photophysical properties, while experimental emission studies over a wide temperature range down to T = 1.5 K lead to better insight into the electronic structures even with respect to spin–orbit coupling efficiencies, radiative rates, and zero-field splitting of the triplet state. All three compounds, with emission quantum yields higher than ϕPL = 70 %, are potentially well suited as emitters for organic light-emitting diodes (OLEDs) based on the singlet-harvesting mechanism. Interestingly, compound1 is by far the most attractive one because of a very small energy separation between the lowest excited singlet S1 and triplet T1 state of ΔE(S1–T1) = 370 cm–1 (46 meV). Such a small value has not been reported so far. It is responsible for the very short decay time of τ(TADF, 300 K) = 3.3 μs. Hence, if focused on the requirements of a short TADF decay time for reduction of the saturation effects in OLEDs, copper(I) complexes are well comparable or even slightly better than the best purely organic TADF emitters.
H. Yersin*, M. J. Leitl, R. Czerwieniec
TADF for singlet harvesting: next generation OLED materials based on brightly green and blue emitting Cu(I) and Ag(I) compounds
Proc. SPIE 2014, 9183, 91830N-1
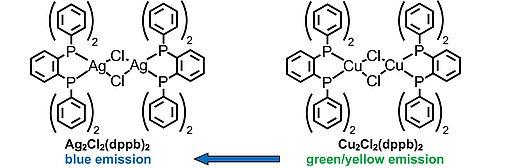
Detailed photophysical studies are presented for Cu2Cl2(dppb)2 and Ag2Cl2(dppb)2. Both compounds show very effective thermally activated delayed fluorescence (TADF) at ambient temperature with an emission quantum yield of the Ag(I) complex of ΦPL(300 K) = 93 %. This emission is blue shifted by 65 nm (2500 cm-1) with respect to the emission of the Cu(I) complex, demonstrating a valuable strategy for engineering blue light emitters. Potentially, these materials are well suited for taking advantage of the singlet harvesting effect in an OLED device. Moreover, both compounds do not show effects of concentration quenching at high emitter concentration, a property which might be attractive for reducing the efficiency roll-off at higher current densities. Investigations down to T = 1.6 K show that spin-orbit coupling (SOC) is particularly weak. This is displayed in the very long emission decay times of the triplet states (T1 states) of metal-toligand charge transfer (3MLCT) character, amounting to τ(Ag2Cl2(dppb)2) = 1.1 ms and τ(Cu2Cl2(dppb)2) = 2.2 ms. According to the TADF mechanism, which leads to the additional decay channel at ambient temperature via the S1 state (of 1MLCT character), an increase of the radiative rate by a factor of 70 and almost 500 for Ag2Cl2(dppb)2 and Cu2Cl2(dppb)2, respectively, is induced. This results in radiative rates at ambient temperature of kr = 6.2 104 s-1 (τr = 16 μs, Ag(I) complex) and 11.7 104 s-1 (τr = 8.5 μs, Cu(I) complex). Simple approaches are presented that allow us to understand the weakness of SOC on the basis of results from DFT and TD-DFT calculations. Investigations of the emission decay properties down to T = 1.6 K further support the conclusions with respect to the SOC strength.
C. L. Linfoot, M. J. Leitl, P. Richardson, A. F. Rausch, O. Chepelin, F. J. White, H. Yersin*, N. Robertson*
Thermally Activated Delayed Fluorescence (TADF) and Enhancing Photoluminescence Quantum Yields of [CuI (diimine)(diphosphine)]+ Complexes - Photophysical, Structural, and Computational Studies
Inorg. Chem. 2014, 53, 10854.
The complexes [Cu(I)(POP)(dmbpy)][BF4] (1) and [Cu(I)(POP)(tmbpy)][BF4] (2) (dmbpy = 4,4′-dimethyl-2,2′-bipyridyl; tmbpy = 4,4′,6,6′-tetramethyl-2,2′-bipyridyl; POP = bis[2-(diphenylphosphino)-phenyl]ether) have been studied in a wide temperature range by steady-state and time-resolved emission spectroscopy in fluid solution, frozen solution, and as solid powders. Emission quantum yields of up to 74 % were observed for 2 in a rigid matrix (powder), substantially higher than for 1 of around 9 % under the same conditions. Importantly, it was found that the emission of 2 at ambient temperature represents a thermally activated delayed fluorescence (TADF) which renders the compound to be a good candidate for singlet harvesting in OLEDs. The role of steric constraints within the complexes, in particular their influences on the emission quantum yields, were investigated by hybrid-DFT calculations for the excited triplet state of 1 and 2 while manipulating the torsion angle between the bipyridyl and POP ligands. Both complexes showed similar flexibility within a ± 10° range of the torsion angle; however, 2 appeared limited to this range, whereas 1 could be further twisted with little energy demand. It is concluded that a restricted flexibility leads to a reduction of nonradiative deactivation and thus an increase of emission quantum yield.
D. A. Kunz, M. J. Leitl, L. Schade, J. Schmid, B. Bojer, U. T. Schwarz*, G. A. Ozin, H. Yersin*, J. Breu*
Quasi-epitaxial Growth of [Ru(bpy)3]2+ by Confinement in Clay Nanoplatelets Yields Polarized Emission
Small 2015, 11, 792.
A nano confinement strategy is presented to control the spatial orientation and emission polarization of phosphorescent metal complexes. Through nano-confinement of the phosphorescent metal complex [Ru(bpy)3]2+ by attaching it to anionic clay nanoplatelets, it is possible to simultaneously lock the spatial orientation of the complex and fix its emission polarization. This quasi-epitaxial approach may provide a future work strategy directed at light emitting diodes and lasers.
M. Stöter, B. Biersack, S. Rosenfeldt, M. J. Leitl, H. Kalo, R. Schobert, H. Yersin*, G. A. Ozin, S. Förster, J. Breu*
Encapsulation of Functional Organic Compounds in Nanoglass for Optically Anisotropic Coatings
Angew. Chem. Int. Ed. 2015, 16, 4963.
A novel approach is presented for the encapsulation of organic functional molecules between two sheets of 1 nm thin silicate layers, which like glass are transparent and chemically stable. An ordered heterostructure with organic interlayers strictly alternating with osmotically swelling sodium interlayers can be spontaneously delaminated into double stacks with the organic interlayers sandwiched between two silicate layers. The double stacks show high aspect ratios of >1000 (typical lateral extension 5000 nm, thickness 4.5 nm). This newly developed technique can be used to mask hydrophobic functional molecules and render them completely dispersible in water. The combination of the structural anisotropy of the silicate layers and a preferred orientation of molecules confined in the interlayer space allows polymer nanocomposite films to be cast with a well-defined orientation of the encapsulated molecules, thus rendering the optical properties of the nanocoatings anisotropic.