Research
Research Topic
Assembly of eukaryotic ribosomes requires four ribosomal RNAs (rRNAs). The nascent ribosomal RNA precursor contains three of these rRNAs and is transcribed by the RNA polymerase (Pol) I machinery.
Using a structural biology hybrid approach and in vitro biochemistry structure-function analyses we study the molecular basis of Pol I transcription and its regulation. In combination with ex vivo and in-cell techniques, we correlate our findings with the in vivo situation.
The RNA polymerase I transcription initiation system
(DFG-funded 'Emmy Noether' project)
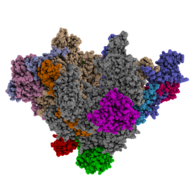
Cryo-EM reconstruction of the yeast RNA polymerase I early initiation intermediate including Rrn3 and Core Factor (Pilsl & Engel, Nature Communications 2020)
Combining X-ray crystallography with recent advances in cryo-electron microscopy (cryo-EM) now allows the application of an integrated structural biology hybrid approach for the analysis of transiently stable, multi-protein DNA/RNA complexes. Continuing and expanding the structural analysis of Pol I and its transcription machinery with this integrated approach allowed us to get insight into the structural basis of transcription initiation (Engel et al., Nature Communications 2016; and Engel et al., Cell 2017). We further analyzes the mechanisms of promoter recognition and DNA-duplex melting (Pilsl and Engel, Nature Communications 2020). Now, we are now interested in understanding the structural and functional basis of Pol I promoter targeting and escape in a close-to-native environment.
Mechanisms of transcription by RNA polymerase I
(SFB 960 project A8)
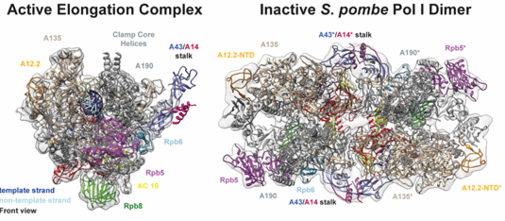
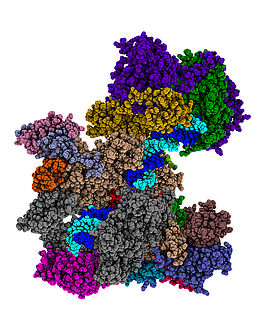
Cryo-EM reconstruction of the S. pombe RNA polymerase I actively elongting complex and inactive Pol I dimer (Heiss, Daiß, Becker and Engel, Nature Communications 2020)
Structural analysis of Pol I and its transcription machinery has revealed striking differences but also many similarities with Pol II and Pol III. This was demonstrated by the 3D structure of the entire 14-subunit, 590 kDa Pol I enzyme from yeast solved by X-ray crystallography (Engel et al., Nature 2013). We are now interested in studying structure-function relationships in Pol I transcription throughout organisms to understand the molecular mechanisms underlying rDNA transcription regulation. Recent work presented three high-resolution single particle cyro-Electron Microscopy (cryo-EM) reconstructions of Pol I from fission yeast including biophysical characterization (Heiss, Daiß, Becker, and Engel, Nature Communications 2021). The analyses demonstrated unexpected conservation of activation and hibernation strategies despite major structural divergence. On the other hand, novel questions about subunit functionality arose, which we tackling momentarily.
The RNA polymerase I transcription cycle
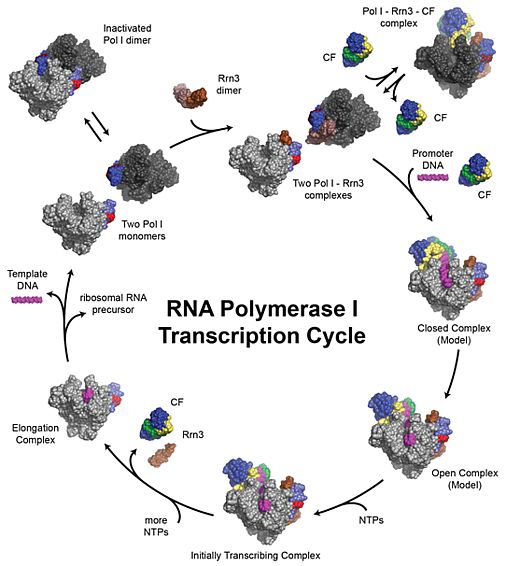
In order to transcribe their target genes, DNA-dependent polymerases undertake three defined steps: Firstly, they engage their template DNA promoter sequence, melt the DNA double strand and start transcription into RNA (initiation). Second, continuous production of the full-length RNA takes place (elongation). Finally, the transcription is stopped, leading to dissociation of template, product RNA and polymerase (termination). Our review describes the Pol I transcription cycle from a structural biology perspective and outlines the specific factors we study (Engel et al., Annual Reviews Biophysics 2018).
The structural basis of human transcription in vitro
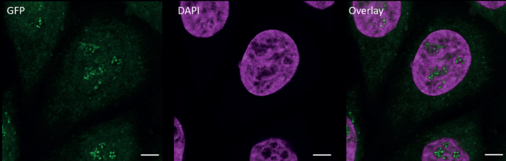 CRISPR/Cas9-generated cell line carrying a homozygous knock-in of cleavable GFP-tag on Pol I and III
CRISPR/Cas9-generated cell line carrying a homozygous knock-in of cleavable GFP-tag on Pol I and III
Since many features of multi-subunit RNA polymerase structure and function are conserved among organisms, detailed in vitro characterization was performed on yeast enzymes due to their availability until recently. However, regulatory mechanisms and pathological phenotypes of the human enzymes can hardly be studied in less complex organisms. To investigate these mechanisms of human transcription in vitro, we used the CRISPR/Cas9 technology to generate a cell line that carries a cleavable GFP-tag on a subunit that is part of Pol I and Pol III (Ramsay* et al., Nature Communications 2020). This allows the purification human Pols I and III for their structural and functional characterization.
 Cryo-EM reconstruction of human RNA polymerase III
Cryo-EM reconstruction of human RNA polymerase III