Precise Nanometer Ruler for Biomolecules
Regensburg researchers take part in a worldwide study
April 21, 2023
How precisely can the movement of biomolecules be measured using light? This question has now been answered in a worldwide blind comparison study. Nineteen laboratories, including the team led by Prof. Dr. Dina Grohmann from the Department of Microbiology at the University of Regensburg, measured the dynamic distance changes of proteins and compared their distance measurements with the results of other groups. The study was initiated and coordinated by Prof. Dr. Thorben Cordes and Prof. Dr. Don C. Lamb of LMU Munich, Prof. Dr. Claus Seidel of HHU Düsseldorf and Dr. Anders Barth of TU Delft and has now been published in the journal Nature Methods.
Proteins are biomolecules that control and carry out vital processes in all cells. Observing them at work is therefore of enormous importance for understanding the functioning of our cells. For their studies, the research teams used a technique known as Förster resonance energy transfer (FRET). In this method, two synthetic dye molecules are incorporated into a protein. When one of these dyes is excited by a laser, its energy can be transferred to the other dye molecule in a non-radiative process. The energy transfer efficiency depends on the distance between the two dye molecules and can therefore be used to detect minimal changes in the protein structure. Using this approach, the researchers were essentially able to observe the protein's "molecular gymnastics".
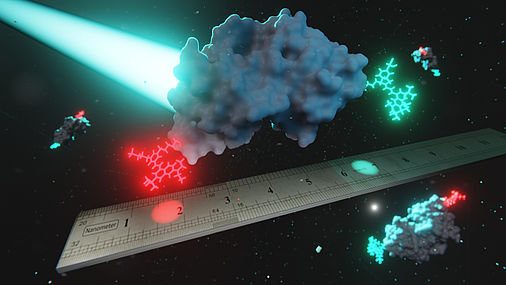
Förster resonance energy transfer is used as a nanometer ruler for proteins. For this purpose, two fluorescent dyes (red, cyan) are incorporated into the protein (white) and the sample is excited with a laser. Photo Credit: © Dr. Kevin Kramm
But how accurate and reproducible is this method? This question arose because there is no standard commercial instrument for this measurement technique. Each research team tends to build its own FRET microscope, and the software used to analyse the data is not standardised either. Therefore, in a blind procedure, the same sample was sent to each of the 19 teams and they were asked to send the analysed data sets back to the organising team without any prior knowledge of the identity of the protein or its structure.
"At the beginning, I was of course worried that our data might be the outlier among the teams. In the end, I was all the more pleased that our results were very close to the average of the study," says Dr Kevin Kramm, who carried out the measurements in Prof Dr Grohmann's laboratory. The research teams were able to detect structural changes in proteins with an accuracy of less than one nanometre and on time scales of less than one millisecond. The results of the study have therefore confirmed the reliability and precision of FRET measurements on protein complexes, adding another versatile tool to the structural biology toolbox. The researchers hope to further refine the method in the future, thereby advancing our understanding of dynamic processes in cells. "This is a fantastic result for the entire FRET community and reinforces the confidence in the measurement method that we have been using and appreciating for years. The study also shows how well international collaboration between different laboratories works," comments Prof. Dr. Dina Grohmann, Head of the Department of Microbiology and the Archaea Centre at the University of Regensburg.
Link to the original publication: https://www.nature.com/articles/s41592-023-01807-0
Information/Contact
Prof. Dr. Dina Grohmann
Department of Microbiology & Archaeenzentrum
Tel.: +49 (0)941 943 3147
E-Mail: dina.grohmann@ur.de