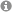Light and nickel simplify chemical reactions
UR researchers develop a method for reliable planning of syntheses
14 June 2023
Cross-coupling reactions–chemical transformations in which two fragments are joined together–are a valuable tool in the synthesis of organic molecules. Applications range from drug development and synthesis of naturally occurring molecules to materials science. Despite many known methods, finding the right conditions for new reactions remained a challenge. Given the numerous factors that can affect the outcome of the reaction, such as the presence or absence of ligand molecules, catalyst precursors, bases, and other additives, optimization is a tedious task. Machine learning and artificial intelligence are promising new approaches to predicting optimal reaction conditions, but training such models also involves significant effort.
A solution to this problem that has now been found by researchers at the University of Regensburg in cooperation with the Zelinisky Institute in Moscow takes a completely different approach: the reaction parameters have been reduced to a minimum and only the two reaction partners that are to be linked are combined with a simple nickel salt and an organic dye under exposure to visible light. No traditional ligands or additives are added to constrain the nickel catalyst (i.e., they provide multiple channels for catalytic reactivity), as is the case in most conventional methods. Under the reaction conditions, a dynamic mixture of many metal complexes is formed, whose electronic state is adjusted by the photocatalyst and the absorbed light energy in such a way that catalytic reactions begin. The principle is comparable to a juggling feat; through which the photocatalyst and the light energy repeatedly bring metal complexes into the catalytically active form, like throwing balls up when juggling. Since light energy is only required to activate the catalysts and these are very reactive without stabilization, energy-efficient, fast reactions are possible. Catalysts that lose their activity (in the image of the juggling feat, these are dropped balls) are continuously repaired by the light energy, so that only extremely small amounts of the catalyst metal, nickel, are needed. The results of years of research have now been published in the scientific journal "Nature".
It was possible to identify reaction conditions for all molecule classes that now allow reliable planning of syntheses. The new reaction principle is referred to as adaptive dynamic homogeneous catalysis, or AD-HoC for short, and makes an important contribution to the development of energy-efficient and effective, and therefore more sustainable, chemical reactions.

Photocatalytic reactions in the laboratory of Prof. König (University of Regensburg) with visible light. © Prof. Dr. Burkhard König
The project has been running for about three years. During this time, numerous experiments were conducted to further develop and confirm the central discovery. The systematic classification of reactants was a breakthrough moment and a particular analytical method of the Russian collaborators, in-situ-mass¬spectrometry, helped to understand the dynamic nature of the catalytic systems. In future work, the concept will now be extended to other metal ions such as copper, cobalt, or iron, and other types of reactions, such as the activation of carbon-hydrogen bonds. Furthermore, the researchers believe that the predictability of the reaction conditions together with the simplicity and efficiency will allow this method to be used in industry either for the synthesis of active pharmaceutical ingredients (APIs), which normally requires a time-consuming series of optimization steps, or for the functionalization of biomolecules or for energy-efficient synthetic transformations on a large scale.
Publication:
I. Ghosh, N. Shlapakov, T. A. Karl, J. Düker, M. Nikitin, J. V. Burykina, V. P. Ananikov, B. König, “General cross-coupling reactions with adaptive dynamic homogeneous catalysis”, Nature 2023, DOI: 10.1038/s41586-023-06087-4.
www.nature.com/articles/s41586-023-06087-4
Information/Contact
Prof. Dr. Burkhard König
Department of Chemistry
Institute of Organic Chemistry
University of Regensburg
Tel.: +49 (0)941 943-4576
E-Mail: burkhard.koenig@ur.de
Web: http://www-oc.chemie.uni-regensburg.de/koenig/index.html