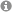Research
Research
Projects
1. Towards a better understanding of maternal mental health: Stress and diet effects on peripartum adaptations (Judith Thomas)
(in collaboration with Prof. Stefan Reber (Unversity of Ulm), Dr Kathi Hillerer and Prof. Ludwig Aigner (both Paracelus University, Salzburg))
Across all mammalian species the peripartum period is characterised by behavioural, neuroendocrine and neuronal adaptations, which prepare a mother for the impending birth and nurturance of the offspring (Brunton and Russell 2008; Slattery and Neumann 2008). While these adaptations are essential to ensure the survival and health of the offspring (Carter et al 2001), we hypothesize that they are also required for maternal mental health (Neumann 2003; Slattery and Neumann 2008). Although anxiolysis, enhanced calmness and attenuated stress-responses of the hypothalamic-pituitary adrenal (HPA) axis are observed in the majority of mothers, a significant percentage display increased vulnerability to mood disorders, such as postpartum anxiety (5-12%; Lonstein 2007), postpartum depression (PPD; 5-25%; Beck 2006) or the much rarer postpartum psychosis (0.1%; Bridges 2008; Jones et al 2008). Despite the high incidence of these disorders, and detrimental outcome for both mother and child, their aetiology remains poorly understood, due in part to the lack of appropriate animal models.
It is feasible that alterations to these normal peripartum adaptations (for more details see Slattery and Neumann, 2008 and Figure 1) caused, for example, by exposure to chronic stress in pregnancy or maternal obesity may underlie postpartum mood disorders.
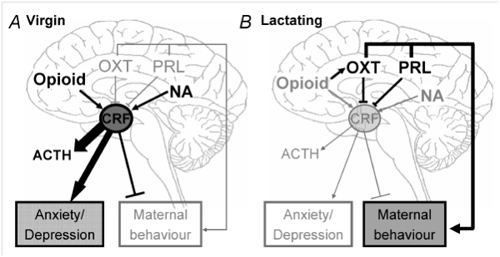
Figure 1: Adapted from Slattery and Neumann, 2008 (J. Physiol)
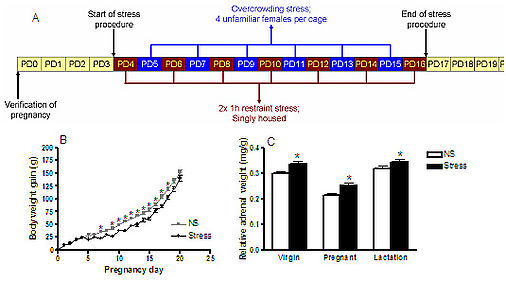
Figure 2: Data demonstrating the effectiveness of the chronic psycho-social stress paradigm
A) Schematic representation of the chronic stress procedure to be applied to pregnant and virgin rats. B) The effect of chronic stress on body weight gain during pregnancy. C) The relative adrenal weight in virgins, late pregnant (PD 20) or lactation day 8 non-stressed and stressed females.
Therefore, the first aim of the project is to characterise the effects of a novel chronic psychosocial stress paradigm (Figure 2) during pregnancy and and high-fat diet exposure throughout the peripartum period on established peripartum adaptations. As such, we hypothesize that exposure to chronic stress in pregnancy will, at least partly, prevent behavioural, neurogenesis, neuroendocrine and/or neuronal adaptations and result in alterations specific to the reproductive status of the female and, therefore, include males/virgins as controls. We will then determine whether genetic differences in anxiety-related behaviour (see Figure 1 in project 2) can protect (low-anxiety females) or worsen (high-anxiety females) the observed consequences of these manipulations. The project is also being extended to reveal the consequences of stress and high-fat diet on adrenal morphology and function. Finally, we will attempt to reverse the detrimental effects of stress exposure via chronic application of antidepressants or selected neuropeptides.
Overall, the research objectives aim to demonstrate a causal link between pregnancy stress and/or diet and postpartum mood and anxiety disorders and to show that innate anxiety levels and key neuropeptides can protect against such stress exposure. The results will also provide novel targets that may be involved in the aetiology and treatment of postpartum mood and anxiety disorders.
These studies are supported by the Deutsche Forschungsgemeinschaft (DFG Ne465/16 and DFG SL141/4-1)
2. Assessment of the contribution of the Neuropeptide S (NPS) system in an animal model of high anxiety-related behaviour
(in collaboration with Dr Gregers Wegener: Aarhus University, Prof. Aleksander Mathe; Karolinska Institute, and Prof. Rainer Landgraf; MPI Munich)
Psychiatric disorders are the most common mental illness with anxiety disorders having a lifetime prevalence of 28.8 % {Kessler et al., 2007). While a number of pharmacotherapies are available, the lack of truly novel-acting compounds has lead in recent years to a more endophenotype-based research approach and a focus on the development of non-GABAergic compounds (Cryan and Slattery, 2007; 2010; Hasler et al., 2006). The large number of neuropeptides, characterized by discrete synthesis sites and widespread receptor distribution, represent likely research candidates for novel therapeutic targets {Landgraf and Neumann, 2004; Slattery and Neumann, 2010).
Neuropeptide S (NPS), originally described in mice, is an example of such a neuropeptide {Xu et al., 2004). Central administration of NPS has been shown to elicit potent anxiolytic and arousal effects in rodents (Pape et al., 2010). In addition to these effects on innate anxiety, direct NPS infusion into the endopiriform nucleus (Meis et al., 2008), or the basolateral amygdala (Jungling et al., 2008), has been demonstrated to facilitate extinction from contextual and conditioned fear, respectively. Intriguingly, human studies have revealed that a single nucleotide polymorphism (SNP) in the NPS receptor (Npsr) is associated with increased risk of panic disorder and over-interpretation of fear (Domschke et al., 2010; Donner et al., 2010; Okamura et al, 2007; Raczka et al., 2010). This A to T polymorphism (rs324981) results in an amino acid substitution from Asn to Ile at position 107 and was originally associated with increased susceptibility for asthma (Laitinen et al, 2004). Although animal studies show that acute NPS application is anxiolytic, the human studies suggest that increased NPS potency is detrimental. It has been suggested that this supposed paradox could be due to an increased level of arousal and over-interpretation of stimuli, which is known to increase the possibility of panic disorder (Domschke et al., 2010).
In order to assess such a hypothesis, it has become apparent that it is important to test novel strategies, and examine the underlying neural mechanisms, in appropriate animal models, which attempt to mimic the human situation. These models generally fall into two main categories; chronic stress paradigms and animals bred for extreme behavioral traits (Cryan and Slattery, 2007). The selective breeding of Wistar (NAB) rats for anxiety-related behavior on the elevated-plus maze (EPM) has resulted in breeding lines of high (HAB) and low (LAB) anxiety-related behaviour, confirmed in numerous tests and laboratories (Figure 1; Neumann, 2010). These in-born differences make these HAB and LAB rodents attractive models to assess the underlying aetiology of affective disorders including the involvement of novel neurotransmitter / neuropeptide systems. Indeed, the differences in anxiety behaviour have been related to SNPs in the vasopressin gene (Murgatroyd et al., 2004). However, whether NPS may also contribute to the anxiety phenotypes of these rodents, or differentially alter behaviour acutely is unknown.
Figure 1: Adapted from Neumann et al., in press (Progress in Neuro-Psychopharmacology & Biological Psychiatry)
Therefore, the aim of the project is to determine whether intracerebral administration of NPS results in differential responses in these lines. Further, we intend to determine if receptor or peptide expression differences, or genetic polymorphisms exist between the lines. Taken together, these studies will reveal the contribution of the NPS system in rodents displaying extremes in anxiety-related behaviour and whether NPS can acutely exert its anxiolytic effects in animals with a genetically-determined psychopathology.
3. Behavioural and central consequences of chronic psychosocial stress
(Anna Schmidtner)
(in collaboration with Prof. Stefan Reber (Universitty of Ulm))
Despite substantial research effort in the last decades, the aetiology of stress-based disorders such as major depression and anxiety remains poorly understood. This has led to a resurgence of interest in developing more relevant animal models than the majority currently employed. Therefore, given the evidence purporting chronic social stress to be a risk factor for the development, not only of cardiovascular and inflammatory diseases, but also of depression and anxiety in vulnerable individuals (Cryan & Slattery 2007), recent attempts have focussed on the development of chronic social stress paradigms (Bartolomucci et al 2003; Berton et al 2006; Engler et al 2005; Fuchs & Flugge 2003; Keeney & Hogg 1999; Reber et al 2006; 2007; Schmidt et al 2007; Stefanski et al 2003). Such paradigms are believed to be more relevant to the human situation than non-social stress paradigms (e.g. repeated restraint; (Cryan & Slattery 2007) and, thus, can better reveal the behavioural consequences of stress exposure.
The majority of these social stressor paradigms have been reported to increase both anxiety- and depression-related behaviour. This is perhaps unsurprising, as there is high co-morbidity between the two disorders (Kennedy 2008). In addition, as in humans, these alterations persist for a long-time after the termination of the stressor (Berton et al 2006; Fuchs 2005; Kennedy 2008; Krishnan et al 2007). Thus, such models have enhanced our knowledge of underlying mal-adaptations caused by chronic stress exposure, and continue to do so. However, they also highlight a drawback of the models currently used to assess stress-related disorders. In order to really dissect the mechanisms underlying anxiety or depression, animal models are needed, which specifically induced one phenotype.
This ongoing project has characterised the behavioural consequences of two established chronic psychosocial stress paradigms in mice; namely chronic subordinate colony housing (CSC) and social defeat/ overcrowding (SD/OC). Both stress paradigms lead to an anxiogenic phenotype; albeit with different temporal profiles and not towards a novel con-specific (social anxiety). CSC exposure elevates anxiety-related behaviour immediately after stressor termination, which lasts for at least 1 week. In contrast, the anxiogenic phenotype only develops 1 week after SD/OC termination. Importantly, neither stress paradigm resulted in an increase in depression-related behaviour as assessed using the forced swim test, tail suspension test and saccharin preference test (see Slattery Publication List). Therefore, both models are unique for uncovering the molecular underpinnings of anxiety-related behaviour without conflicting depression-based alterations.
Therefore, in the present study we assessed the impact of these two well-validated, clinically-relevant, psychosocial stress paradigms on short- and long-term anxiety- and depression-related behaviour.
4. Novel strategies for the optimized treatment of depression (Anna Schmidtner)
Major depressive disorder (MDD) imposes a huge burden on both patients and society as it is a chronic severe and disabling disease. Treatment for MDD started approximately 60 years ago with the first antidepressants (AD) and evolved quickly in its options but still bears some limitations. Beside distinct side effects, the onset of action of AD usually requires several weeks of treatment which often leads to a lack of compliance in patients with 30 % not responding to treatment at all. Thus, an optimized AD treatment and novel AD strategies are desperately needed.
In this context, my main research interest is to reveal detailed neuro-behavioural mechanisms underlying MDD in validated animal models like inherent anxiety or chronic psychosocial stress in a gender-dependent context to optimize AD treatment. Further, I aim to identify new targets relevant for AD treatment by analysing changes in expression patterns of specific markers during chronic stress and drug treatment as well as test the effectiveness of novel ADs by means of behavioural and molecular techniques combined with pharmacological and/ or genetic manipulation.
These studies are supported by the BMBF (OPTiMD).
Profile
Profile
Since 04/2013
Akad. Rat. a.Z., University of Regensburg
04/2013
Completed Habilitation at the University of Regensburg
04/2011 – 03/2013
Acting Professor of Neurophysiology, Institute of Zoology. University of Regensburg
2010
Commenced Habilitation at the University of Regensburg
2009 - 2011
Akad. Rat. a.Z. University of Regensburg, Institute of Zoology. University of Regensburg
2006 – 2009
Senior Post-doctoral Researcher (ELITE-Network Bavaria funded)
Institute of Zoology, University of Regensburg
2004 – 2006
NIH-Funded Post-doctoral Researcher, Novartis Institutes for Biomedical Research, Basel, Switzerland: “The development of a novel translational animal model of depression”. Supervisor: Dr J.F. Cryan
1999 – 2003
BBSRC Industrial CASE Award PhD Studentship, University of Bristol and Organon Laboratories Ltd, Newhouse, Scotland: “Characterisation of a novel antidepressant: Org34167”. Supervisors: Prof D.J. Nutt, Dr A.L. Hudson, Dr D. Hill
1995 - 1999
Bachelor of Science (Honours) in Pharmacology, University of Glasgow (2,1)
Awards and Achievements:
2015: Elected host of an ECNP Research Internship
2015: Co-organiser of German Neuropeptide Symposium
2014: External PhD examiner (Nicoletta Nava, Aarhus University, Denmark)
2013: Cover illustration of June issue of Hippocampus
2013: External PhD examiner (Daniela Felice, University College Cork, Ireland)
2013: Co-organiser of Parental Brain 2013 congress
since 2011 Editor PLoS ONE
since 2011 Member of ECNP Workshop Committee
since 2011 Editor Acta Neuropsychiatrica
since 2010 Review Editor Frontiers in Psychopharmacology
since 2010 ECNP Ordinary Member
since 2009: Associate Editor BMC Neuroscience
2009: Participant in ECNP Targeted Expert Meeting on Anxiety Disorders and Anxiolytics, Istanbul, Turkey
2007: Post-doctoral Poster Prize winner Parental Brain Conference, Boston, USA
2005: Travel award to ECNP young scientist Workshop, Nice, France
Grants Awarded:
BMBF: Novel strategies for the optimized treatment of depression; OPTiMD: Co-Investigator.
Funding period: 36 months beginning 10/2014
Total awarded €639, 000
DFG: SL141/4-1 "Stress in females", Principle Investigator.
Funding period: 48 months beginning 09/2011. Total awarded €295, 350
Research Interests:
1. The role of neuropeptides in anxiety- and affective- disorders
2. The neural circuitry underlying behavioural responses relevant to stress, reward, depression and anxiety
3. The molecular basis of Social Anxiety Disorder and the assessment of novel treatment options
4. The aetiology and treatment of post-partum psychiatric disorders
5. Sex-differences in the aetiology and treatment of stress-related disorders
Teaching Experience:
| 2015 | Two-day workshop at the University of Tartu, Estonia, on “Behavioural approaches to study psychiatric disorders in rodents.” |
| since 2010 | Lecturer in “Animal Physiology” and “Neurobiology” Undergraduate Lecture Series |
| since 2006 | Bachelor and Master students method practica instructor/demonstrator |
| since 2006 | Lecturer in international ELITE Masters course in Experimental and Clinical Neurosciences |
| since 2006 | Research and Thesis Supervisor for undergraduate, Bachelor, Masters and PhD students |
| 1999 - 2003 | Tutor in Pharmacology laboratory practica (University of Bristol) |
(Co)Supervision of PhD students
2007 – 2010 Katharina Hillerer – Graduated magna cum laude
2009 – 2012 Iulia Toth – Graduated summa cum laude
2010 – 2014 Benjamin Jurek; Stefanie Martinetz; Clara Perani
All graduated magna cum laude
2014 - Anna Schmidtner
Professional Skills:
Animal handling, husbandry and welfare: approved for animal research in the U.K., Switzerland and Germany
Biochemical and molecular biology techniques: cryostat sectioning; densitometric image analysis; immunohistochemistry; in situ hybridization; microarray analysis; radioligand binding; receptor autoradiography; RT-PCR; western blotting
Endocrinology: ELISA and radioimmune assays for corticosterone, ACTH, oxytocin and vasopressin; in-vivo blood sampling via indwelling jugular-vein catheter
Behavioural testing (rat and mice unless specified): cued- and contextual-fear conditioning; elevated plus maze; forced swim test; intracranial self-stimulation responding (rats); light-dark box; progressive ratio for sucrose responding; monitoring maternal behaviour; novelty-suppressed feeding; open field test; punished drinking test (Vogel test); rotarod; sucrose preference testing; tail suspension test (mice); social interaction test; stress-induced hypothermia (mice),Surgical procedures: bilateral olfactory bulbectomy; intra-cardiac perfusion; jugular-vein catheterisation; microdialysis; stereotaxic surgery
Ad-hoc reviewer:
For over 25 journals including Biological Psychiatry, Molecular Psychiatry, Neuropharmacology, Neuropsychopharmacology, Neuroscience and Biobehavioral Reviews and Psychopharmacology
Research Collaborations:
Prof. Ludwig Aigner (Paracelsus Uni. Salzburg, Austria)
Dr John Cryan (Uni. College Cork, Ireland)
Dr Kathi Hillerer (Paracelsus Uni. Salzburg, Austria)
Prof. Aleksander Mathe (Karolinska Institute)
Prof. Maurizio Popoli (Uni. of Milan, Italy)
Prof. Stefan Reber (Uni. Ulm, Germany)
Prof. Nicolas Singewald (Uni. Innsbruck, Austria)
Prof. Per Svenningson (Karolinska Institute, Sweden)
Dr Gregers Wegener (Aarhus Uni., Denmark)
Language Skills:
English (mother tongue), German (intermediate), Hungarian (basic)
Other:
My current h index is 23 with a total citation count of 1923 (correct as of November 2015):
(see https://scholar.google.co.uk/citations?user=51uR5VIAAAAJ&hl=en for up to date information)
Publications
Publications
David Slattery – Publication List November 2015
Reviewed Articles
Perani, C.V., Neumann, I.D., Reber, S.O. & Slattery, D.A. - High Fat Diet Induces Maladaptive Adrenal Gland Plasticity During the Peripartum Period. Scientific Reports 5:14821. doi: 10.1038/srep14821 (2015)
Slattery, D.A., Uzunov, D.P. & Cryan, J.F. - 11-beta-hydroxysteroid type 1 knockout mice display an antidepressant-like phenotype. Acta Neuropsychiatrica Sep 24:1-6. [Epub ahead of print] (2015)
Jurek, B., Slattery, D.A., Liu, Y., Neumann, I.D., Aguilera, G. & van den Burg, E.H. - Oxytocin regulates stress-induced CRF gene transcription through CREB-regulated transcription coactivator 3 (CRTC3). Journal of Neuroscience 35:12248-60 (2015)
Slattery, D.A., Naik, R., Grund, T., Yen, Y-C., Sartori, S.B., Füchsl, A.M., Finger, B.C., Elfving, B., Nordemann, U., Guerrini, R., Calo’, G., Wegener, G., Mathé, A.A., Singewald, N., Czibere, L., Landgraf, R. & Neumann, I.D. - Selective breeding for high anxiety introduces a synonymous SNP that increases neuropeptide S receptor activity. Journal of Neuroscience 35: 4599-4613 (2015)
Neumann, I.D & Slattery, D.A. - Oxytocin in general anxiety and social fear: A translational approach. Biological Psychiatry doi: 10.1016/j.biopsych.2015.06.004 (2015)
Langgartner, D., Füchsl, A.M., Uschold-Schmidt, N., Slattery, D.A. & Reber, S.O. - Chronic subordinate colony housing paradigm: a mouse model to characterize the consequences of insufficient glucocorticoid signaling. Frontiers in Psychiatry doi: 10.3389/fpsyt.2015.00018 (2015)
Bosch, O.J., Slattery, D.A. & Neumann, I.D. - The 5th Parental Brain Conference. Journal of Neuroendocrinology 26: 625-6 (2014)
Zoicas, I., Slattery, D.A. & Neumann, I.D. - Brain oxytocin in social fear conditioning and its extinction: Involvement of the lateral septum Neuropsychopharmacology 39: 3027-35 (2014)
Hillerer, K.M., Neumann, I.D., Couillard-Despres, S., Aigner, L. & Slattery, D.A. - Lactation-induced reduction in hippocampal neurogenesis is reversed by repeated stress exposure. Hippocampus 24: 673-83 (2014)
Slattery, D.A. & Cryan, J.F. - The ups and downs of modelling mood disorders in rodents. ILAR Journal 55: 297-309 (2014)
Perani, C.V. & Slattery, D.A. - Using animal models to study postpartum psychiatric disorders. British Journal of Pharmacology 171: 4539-55 (2014)
Galea, L.A.M., Leuner, B. & Slattery, D.A. - Hippocampal plasticity during the peripartum period: Influence of sex steroids, stress and ageing. Journal of Neuroendocrinology 26: 641-8 (2014)
Peters, S., Slattery, D.A., Uschold-Schmidt, N., Reber, S.O. & Neumann, I.D. - Dose-dependent effects of chronic central infusion of oxytocin on anxiety, chronic stress-related parameters and oxytocin receptor binding in mice. Psychoneuroendocrinology 42: 225-36 (2014)
Hillerer, K.M., Neumann, I.D., Couillard-Despres, S., Aigner, L. & Slattery, D.A. - Sex-dependent regulation of hippocampal neurogenesis under basal and chronic stress conditions in rats. Hippocampus 23: 476-487 (2013)
Peters, S.#, Slattery, D.A.#, Flor, P.J., Neumann, I.D. & Reber, S.O. - Differential effects of baclofen and oxytocin on the increased ethanol consumption following chronic psychosocial stress in mice. Addiction Biology 18: 66-77 (2013)
Hillerer, K., Neumann, I.D., & Slattery, D.A. - From stress to postpartum mood disorders: How chronic peripartum stress can impair maternal adaptations. Neuroendocrinology 95: 22-38 (2012)
Bartlang, M.S., Neumann, I.D., Slattery, D.A., Uschold, N., Kraus, D., Helfrich-Förster, C. & Reber, S.O. - Time matters: Pathological effects of repeated psychosocial stress during the active, but not inactive, phase of male mice. Journal of Endocrinology 215: 425-437 (2012)
Jurek, B.#, Slattery, D.A.#, Maloumby, R., Hillerer, K., Koszinowski, S., Neumann, I.D., van den Burg, E.H. - Differential contribution of hypothalamic MAPK activity to anxiety-like behaviour in virgin and lactating rats. PLoS ONE 7: art. no. e37060 (2012)
Okimoto, N., Bosch, O.J., Slattery, D.A., Pflaum, K., Matsushita, H., Wei, F-Y., Ohmori, M., Nishiki, T., Ohmori, I., Hiramatsu, Y., Matsui, H., Neumann, I.D. & Tomizawa, K. - RGS2 mediates the anxiolytic effect of oxytocin. Brain Research 1453: 26-33 (2012)
Toth, I., Neumann, I.D. & Slattery, D.A. - Central administration of oxytocin receptor ligands affects cued fear extinction in rats and mice in a timepoint-dependent manner. Psychopharmacology 223: 149-158 (2012)
Slattery, D.A. & Cryan, J.F. - Using the rat forced swim test to assess antidepressant-like activity in rodents. Nature Protocols 7: 1009-14 (2012)
Toth, I., Neumann, I.D. & Slattery, D.A. - Social fear conditioning: A novel and specific animal model to study Social Anxiety Disorder. Neuropsychopharmacology 37: 1433-43 (2012)
Toth, I., Dietz, M., Peterlik, D., Huber, S.E., Fendt, M., Neumann, I.D., Flor, P.J., & Slattery, D.A. - Pharmacological interference with metabotropic glutamate receptor subtype 7 but not subtype 5 differentially affects within- and between-session extinction of Pavlovian conditioned fear. Neuropharmacology 62: 1619-26 (2012)
Slattery, D.A., Uschold, N., Magoni, M., Baer, J., Popoli, M., Neumann, I.D & Reber, S.O. - Behavioural consequences of chronic psychosocial stress: Anxiety without depression. Psychoneuroendocrinology 37: 702-14 (2012)
Wegener, G., Finger, B.C., Elfving, B., Keller, K., Singewald, N., Slattery, D.A., Neumann, I.D. & Mathé, A.A. - Neuropeptide S alters anxiety, but not depression-like behavior in the Flinders Sensitive Line rats, a genetic animal model of depression. International Journal of Neuropsychopharmacology 9: 1-13 (2012)
Hillerer, K., Reber, S.O., Neumann, I.D., Slattery, D.A. - Exposure to chronic pregnancy stress reverses peripartum-associated adaptations: Implications for postpartum anxiety and mood disorders. Endocrinology 152: 3930-40 (2011)
Neumann, I.D., Wegener, G., Homberg, J.R., Cohen, H., Slattery, D.A., Zohar, J., Olivier, J.D.A. & Mathé, A.A. - Animal models of depression and anxiety: What do they tell us about the human condition? Progress in Neuro-Psychopharmacology & Biological Psychiatry 35: 1357-75 (2011)
Lukas, M., Toth, I., Reber, S.O., Slattery, D.A., Veenema, A.H., & Neumann, I.D. - The neuropeptide oxytocin facilitates prosocial behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology 36: 2159-68 (2011)
Reber, S.O., Peters, S., Slattery, D.A., Hofmann, C., Schoelmerich, J., Neumann, I.D., & Obermeier, F. - Mucosal immunosuppression and epithelial barrier defects are key events in murine psychosocial stress-induced colitis. Brain, Behavior and Immunity 25: 1153-61 (2011)
Slattery, D.A., Neumann, I.D. & Cryan, J.F. - Transient inactivation of the infralimbic cortex induces antidepressant-like effects in the rat. Journal of Psychopharmacology 25: 1295-303 (2011)
Slattery, D.A. & Neumann, I.D. - Chronic icv oxytocin attenuates the pathological high anxiety state of selectively bred Wistar rats. Neuropharmacology 58: 56-61 (2010)
Cryan, J.F. & Slattery, D.A. GABAB receptors and depression: Current status. Advances in Pharmacology 58: 427-51 (2010)
Slattery, D.A. & Neumann, I.D. - Oxytocin and major depressive disorder: Experimental and clinical evidence for links to aetiology and possible treatment. Pharmaceuticals 3: 702-724 (2010)
Slattery, D.A. & Neumann, I.D. No stress please! Mechanisms of stress hyporesponsiveness of the maternal brain. Journal of Physiology 586: 377-385 (2008)
Bosch, O.J., Müsch, W., Bredewold, R., Slattery, D.A. & Neumann, I.D. - Prenatal stress increases HPA axis activity and impairs maternal care in lactating female offspring: Implications for postpartum mood disorder. Psychoneuroendocrinology 32: 267-78 (2007)
Slattery, D.A., Markou, A. & Cryan, J.F. - Evaluation of reward processes in an animal model of depression. Psychopharmacology 190: 555-568 (2007)
Cryan, J.F. & Slattery, D.A. - Animal models of mood disorders: recent developments. Current Opinion in Psychiatry 20: 1-7 (2007)
Slattery, D.A. & Cryan, J.F. - The role of GABAB receptors in depression and antidepressant-related behavioural responses. Drug Development Research 67: 477-494 (2006)
Slattery, D.A., Markou, A., Froestl, W. & Cryan, J.F. - The GABAB receptor-positive modulator GS39783 and the GABAB receptor agonist baclofen attenuate the reward-facilitating effects of cocaine: Intracranial self-stimulation studies in the rat. Neuropsychopharmacology 30: 2065-72 (2005)
Slattery, D.A., Morrow, J.A., Hudson, A.L., Hill, D.R., Nutt, D.J. & Henry, B. - Comparison of alterations in c-fos and Egr-1 (zif268) expression throughout the rat brain following acute administration of different classes of antidepressant compounds. Neuropsychopharmacology 30: 1278-87 (2005)
Slattery, D.A., Desrayaud, S. & Cryan, J.F. - GABAB receptor antagonist-mediated antidepressant-like behavior is serotonin-dependent. Journal of Pharmacology and Experimental Therapeutics 312: 290-296 (2005)
Slattery, D.A., Hudson, A.L. & Nutt, D.J. - The evolution of antidepressant mechanisms. Fundamental and Clinical Pharmacology 18: 1-22 (2004)
Sumner, B.E., Cruise, L., Slattery, D.A., Hill, D.R., Shahid, M. & Henry, B. - Testing the validity of c-fos expression profiling to aid the therapeutic classification of psychoactive drugs. Psychopharmacology 172: 306-321 (2004)
Book Chapters:
Slattery, D.A. & Cryan, J.F. - Animal models of depression – where are we going? Depression: From Psychopathology to Pharmacotherapy. Vol 27: 124-138. Editors: Cryan, J.F. & Leonard, B.E., Karger AG, Basel. (2010)
Cryan, J.F. & Slattery, D.A. - GABA receptors and depression: Focus on GABAB. Recent developments on depression research; Research Signpost. Editors Shirayama, Y. & Chaki, S. (2009)
People
People
PhD Students:
Anna Schmidtner
Master Students:
Judith Thomas