Unpacking the smart way
How Incoming adenoviruses change their chromatin structure for efficient gene expression
1 September 2023
Adenoviruses, known for their low pathogenicity and technological approachability, have become instrumental in many therapeutic applications, including as vaccination vector platform during the recent SARS-CoV-2 pandemic. Central to their efficacy is the remarkably efficient delivery and expression of their genetic material.
Two scientific teams led by Gernot Laengst from the University of Regensburg and Harald Wodrich from the University of Bordeaux have obtained insight into this process in unprecedented detail.
Employing advanced microscopy techniques and genome structure analyses, they traced the early stages of adenoviral infection in human cells. This approach allowed a detailed description of the viral genome intricately wrapped with the viral pVII protein within the incoming virion. Furthermore, their investigation revealed the transformation of the viral chromatin structure, converting the densely packed genome into an exceptionally efficient template for gene expression as it traverses from the cell surface to the cell nucleus.
The scientist could show that in the virus, the viral DNA is tightly wrapped around the viral pVII protein. This unique conformation facilitates seamless accommodation of the genome within the confined viral capsid. Once viruses entered the cell, the packaged viral DNA completes its journey to the nucleus in approximately 30 minutes. Following capsid release and nuclear import parts of the viral genome alter the transport configuration and open up. During this process, a few pVII proteins are specifically removed and replaced by cellular histone proteins at the regulatory sites of early activated viral genes. This strategic exchange with histone proteins unlocks the potential for the viral genetic information to hijack the cellular transcription machinery, enabling efficient gene expression – a process that starts around thirty minutes after the genome arrives in the nucleus, culminating in cell infection.
By comprehending the intricate structural dynamics of the adenoviral genome during the initial stages of infection, the researchers aim to leverage this knowledge for the enhancement of adenoviral vector design. This holds promise for refining applications such as vaccination and gene therapy, thereby elevating both safety and efficiency.
The study was recently published in the Journal of the European Molecular Biology Organization (EMBO Journal).
Original publication:
"Changes in adenoviral chromatin organization precede early gene activation upon infection", Schwartz, U.; Komatsu, T.; Huber, C.; Lagadec, F.; Baumgartl, C.; Silberhorn E.; Nützel M.; Rayne, F.; Basyuk E.; Bertrand E.; Rehli M.; Wodrich H.; Längst, G.; The EMBO Journal (2023) e114162.
https://doi.org/10.15252/embj.2023114162
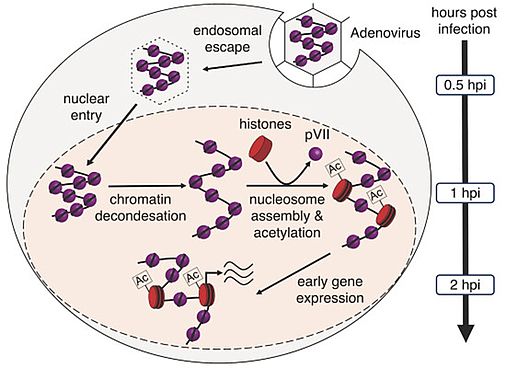
The genome of the adenovirus is specifically packaged with the viral protein pVII. After the virus enters the cell, the genome is released from the viral envelope and transported into the cell nucleus. Before the viral information can be read, protein pVII is specifically exchanged at regulatory important sites of the viral genome by cellular packaging proteins (histones). After chemical modification of the histones (acetylation), the cellular machinery for reading the viral information is hijacked and the virus can multiply. Illustration © Dr. Uwe Schwartz
Information/Contact
Prof. Gernot Längst, Biochemistry III
University of Regensburg
E-Mail: gernot.laengst@ur.de
Prof. Harald Wodrich
Microbiologie Fondamentale et Pathogénicité,
Université de Bordeaux, France
E-Mail: harald.wodrich@u-bordeaux.fr




