Publications of the RTG
2025
66.
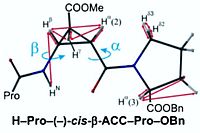
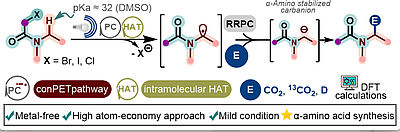
K. Vega, A, Sánchez, T. Mandal, W. Silva, R. Gschwind, B. Konig, M. Paixão, ACS Catal. 2025, 15, 18087–18096 "Synthesis of α-Amino Acids by ConPET-mediated CO2 Fixation into Amides"
65.
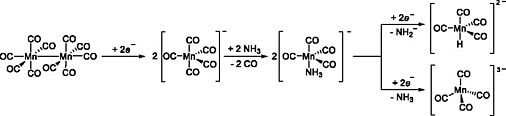
F. Wieberneit, N. Korber, Eur. J. Inorg. Chem. 2025, 28, e202500401, Carbonyl Metalates in Liquid Ammonia: Reduction of Mn2(CO)10 down to [Mn(CO)4]3−
DOI: 10.1002/ejic.202500401
Start of the Second Period
64.
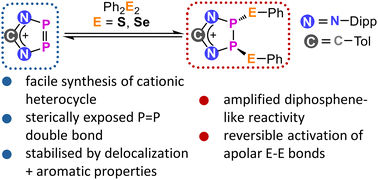
J. Wieneke, F. Cirigliano, M.Schorpp, Chem. Commun., 2025, 61, 17601-17604, "A diazadiphospholenium cation featuring a reactive P[double bond, length half m-dash]P bond: synthesis and reversible main-group bond activation"
DOI: 10.1039/D5CC04820F
63.
M. Kümper, F. F. Westermair, T. Götz, R. M. Gschwind, J. O. Bauer, Angew. Chem. Int. Ed. 2025, 64, e202517017 "Si−H Activation via Dynamic Permutational Isomerism: A Ligand-Directed Route to Dehydrogenative Coupling"
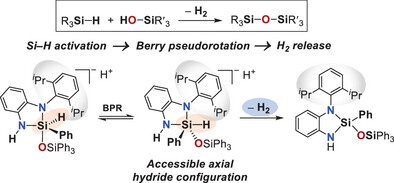
62.
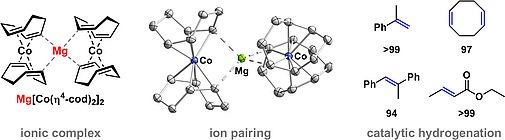
M. Gawron, J. Eder, X. Weichselgartner, R. M. Gschwind, R, Wolf, Organometallics. 2025, 44, 2141–2145, "Flash Communication: A Highly Reduced Magnesium Dicobalt Complex for the Hydrogenation of Tri- and Tetra Substituted Alkenes"
DOI: 10.1021/acs.organomet.5c00246
61.
L. Zimmermann, R. Szlosek, C. Scholtes, C. Riesinger, L. Dütsch, R. M. Gschwind, M. Scheer. Chem. Europe. 2025, 3, e202500116, "Pnictogenium Ions as a Powerful Tool for the Synthesis of Three- and Five-Membered Interpnictogen Chains"

60.
C. L. Scholtes, J. Ilgen, R. M. Gschwind, Chem. Commun. 2025, 61, 6921-6924, "Efficient detection of 1H{,} 15N correlations in hydrogen bonded low molecular catalyst–substrate intermediates without selective 15N-labelling"
DOI: 10.1039/D5CC00537J

59.
E. K. Taskinen, D. Kolb, M. Morgenstern, B. König, Chem. Eur. J. 2025,31, "Photocatalyzed Dehydration of 1-Aryl-1,2-Ethanediols to Methyl Ketones Driven by Eosin Y Fragmentation Products"
DOI: 10.1002/chem.202404200

58.
Y. Liu, F. Westermair, I. Becker, S. Hauer, M. Bodensteiner, C. Hennig, G. Balazs, F. Meyer, R. Gschwind, R. Wolf, J. Am. Chem. Soc. . 2025, "Synthesis and Reactivity of an Iron-Tin Complex with Adjacent Stannylidyne and Ferriostannylene Units"
DOI: 10.1021/jacs.4c18423
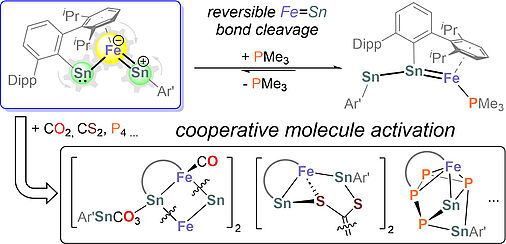
57.
N. Sülzner, G. Jung, P. Nuernberger, Chemical Science . 2025, 16, 1560-1596 "A dual experimental-theoretical perspective on ESPT photoacids and their challenges ahead"
DOI: 10.1039/D4SC07148D

56.
N. Schubert, J. W. Southwell, M. Vázquez-Hernández, S. Wortmann, S. Schloeglmann, A.-K. Duhme-Klair, P. Nuernberger, J. E. Bandow, N. Metzler-Nolte RSC Chem. Biol. 2025, 5, 1201-1213 "Fluorescent probes for investigating the internalisation and action of bioorthogonal ruthenium catalysts within Gram-positive bacteria"
DOI: 10.1039/D4CB00187G

55.
T. Huber, G. Mayer, M. Kümper, W. Silva, N. Fontana, A. Falk, S. H. F. Schreiner, J. Gramüller, A. Scrimgeour, E. Groß, R. M. Gschwind, D. Horinek, P. Nuernberger, J. O. Bauer* Angew. Chem. Int. Ed. 2025, 64, e202425049, "Where Does the Proton Go? Structure and Dynamics of Hydrogen-Bond Switching in Aminophosphine Chalcogenides"
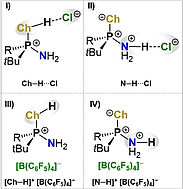
54.
V. Burger, M. Franta, A. C. O‘Donoghue, A.R. Ofial, R. M. Gschwind, H. Zipse, J. Org. Chem., 2025, 90, 2298–2306, "Pyridinamide Ion Pairs: Design Principles for Super-Nucleophiles in Apolar Organic Solvents"
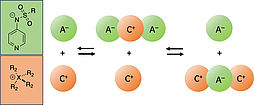
53.
V. Burger, M. Franta, A.R. Ofial, R. M. Gschwind, H. Zipse , J. Am. Chem. Soc., 2025, 147, 5043–5050 "Highly Nucleophilic Pyridinamide Anions in Apolar Organic Solvents due to Asymmetric Ion Pair Association"
DOI: 10.1021/jacs.4c14825
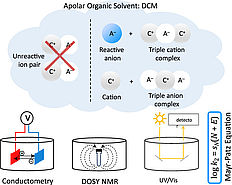
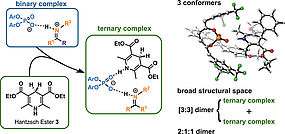
52. M.Franta, A. Pattanaik, W. Silva, K. Motiram-Corall, J. Rehbein, R. M. Gschwind, J. Am. Chem. Soc., 2025, 147, 2549–2558 „The Elusive Ternary Intermediates of Chiral Phosphoric Acids in Ion Pair Catalysis-Structures, Conformations, and Aggregation”
DOI: 10.1021/jacs.4c14096
2024
51. S. Grotjahn, B. König, Chem. Commun., 2024, 60, 12951-12963 „Common Ground and Divergence: OLED Emitters as Photocatalysts”
DOI:10.1039/D4CC04409F

50. F. An, J. Brossette, H. Jangra, Y. Wei, M. Shi, H. Zipse, A. R. Ofial, Chem. Sci., 2024, 15, 18111-18126 „Reactivities of tertiary phosphines towards allenic, acetylenic, and vinylic Michael acceptors”
DOI:10.1039/d4sc04852k

49. A. Ratzenböck, M. Kobras, A. Rustler,O. Reiser*, Chem. Eur. J., 2024, 30, „Lewis Acid Catalyzed Cyclopropane Ring-Opening-Cyclization Cascade Using Thioureas as a N,N-bisnucleophile: Synthesis of Bicyclic Furo-, Pyrano-, and Pyrrololactams via a Formal [4+1]-Addition”
DOI: 10.1002/chem.202401332

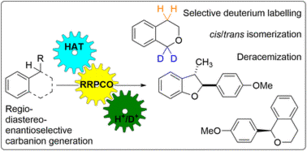
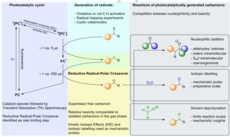
48. S. Grotjahn, L. Müller, A. Pattanaik, A. Falk, G. Barison, J. O. Bauer, J. Rehbein, R. M. Gschwind, B. König, Org. Chem. Front., 2024, 11, 5890-5900. „Regio-, diastereo- and enantioselectivity in the photocatalytic generation of carbanions via hydrogen atom transfer and reductive radical-polar crossover”
DOI: 10.1039/D4QO01219D
47. Y.-M. Tian, X. Pu, A. H. Sánchez, W. Silva, R. M. Gschwind, B. König, Adv. Synth. Catal., 2024, 367. DOI: 10.1002/adsc.202400547; „Photoinduced radical borylation of robust carbon−heteroatom bonds”
DOI:10.1002/adsc.202400547

46. D. J. Scott, J. Cammarata, F. Westermair, P. Coburger, D. Duvinage, M. Janssen, M. K. Uttendorfer; J. Beckmann, R. M. Gschwind, R. Wolf, Angew. Chem. Int. Ed.. 2024, 136, e202408423; „Unravelling White Phosphorus: Experimental and Computational Studies Reveal the Mechanisms of P4 Hydrostannylation”
DOI: 10.1002/anie.202408423

45. S. Yakubov, B. Dauth, W. J. Stockerl, W. da Silva, R. M. Gschwind, J. P. Barham, ChemSusChem. 2024, 17, e202401057; „Protodefluorinated Selectfluor® Aggregatively Activates Selectfluor® for Efficient Radical C(sp3)−H Fluorination Reactions”
DOI: 10.1002/cssc.202401057

44. J. Eder, A. Antonov, E. Tupiklia, R. M. Gschwind, Chem. Eur. J. 2024, 30, e202401793; „Chiral Diselenophosphoric Acids for Ion Pair Catalysis: A Novel Approach to Enhance Both Proton Donating and Proton Accepting Properties”
DOI: 10.1002/chem.202401793

43. M. Hecht, P. Dullinger, W. Silva, D. Horinek, R. M. Gschwind, Chem. Sci. 2024, 15, 9104-9111; „Highly Acidic N-Triflylphosphoramides as Chiral Brønsted Acid Catalysts: The Effect of Weak Hydrogen Bonds and Multiple Acceptors on Complex Structures and Aggregation”
DOI: 10.1039/D4SC01939C

42. T. Huber, J. Bauer, Chem. Eur. J. 2024, 30, e202303760; „A Powerful P−N Connection: Preparative Approaches, Reactivity, and Applications of P-Stereogenic Aminophosphines”
DOI: 10.1002/chem.202303760

41. V. R. Landaeta, T. M. Horsley Downie, R. Wolf, Chem. Rev. 2024, 124, 1323-1463; „Low-Valent Transition Metalate Anions in Synthesis, Small Molecule Activation, and Catalysis”
DOI: 10.1021/acs.chemrev.3c00121
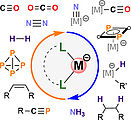
40. M. Gawron, F. Gilch, D. Schmidhuber, J. A. Kelly, T. M. Horsley Downie, A. Jacobi von Wangelin, J. Rehbein, R. Wolf, Angew. Chem. Int. Ed. 2024, 63, e202315381; „Counterion Effect in Cobaltate-Catalyzed Alkene Hydrogenation”
DOI: 10.1002/anie.202315381
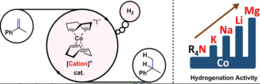
39. S. Grotjahn, C. Graf, J. Zelenka, A. Pattanaik, L. Müller, R. J. Kutta, J. Rehbein, J. Roithová, R. M. Gschwind, P. Nuernberger, B. König, Angew. Chem. Int. Ed. 2024, 63, e202400815; „Reactivity of Superbasic Carbanions Generated via Reductive Radical-Polar Crossover in the Context of Photoredox Catalysis”
DOI: 10.1002/anie.202400815
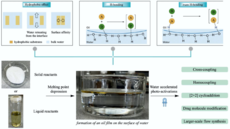
38. Y.-M. Tian, W. Silva, R. M. Gschwind, B. König, Science 2024, 383, 750-756; „Accelerated Photochemical Reactions at Oil-Water Interface Exploiting Melting Point Depression”
DOI: 10.1126/science.adl3092
2023
37. P. Dullinger, D. Horinek, J. Am. Chem. Soc., 2023, 145, 24922-24930; „Solvation of Nanoions in Aqueous Solutions”
DOI: 10.1021/jacs.3c09494

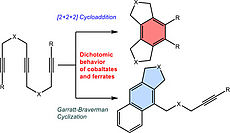
36. B. N. Baumann, H. Lange, F. Seeberger, P. Büschelberger, R. Wolf, M. Hapke, Mol. Catal., 2023, 550, 113482; „Cobalt and iron metallates as catalysts for cyclization reactions of diynes and triynes: [2+2+2] Cycloaddition vs. Garratt-Braverman reaction”
DOI: 10.1016/j.mcat.2023.113482
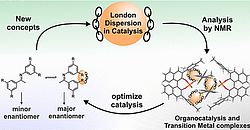
35. J, Gramüller, R. M. Gschwind, Acc. Chem. Res., 2023, 56, 2968-2979; „An NMR Spectroscopy View on London Dispersion in Catalysis: Detection, Quantification and Application in Ion Pair and Transition Metal Catalysis”
DOI: 10.1021/acs.accounts.3c00431
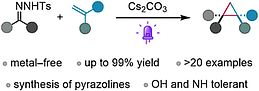
34. V. George, B. König, Chem. Commun., 2023, 59, 11835-11838; „S Photogenerated donor–donor diazo compounds enable facile access to spirocyclopropanes”
DOI: 10.1039/D3CC03581F
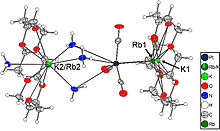
33. P. A. Braun, F. Westermair, R. M. Gschwind, N. Korber, Z. Anorg. Allg. Chem., 2023, 649, e20230017; „(K,Rb)@([2.2.2]crypt)]2(K,Rb)4[Si9W(CO)4] ⋅ 13.4 NH3 – The First Tungsten Functionalized Silicon Zintl Cluster”
DOI: 10.1002/zaac.202300117
32. L. Wylie, J. P. Barham, B. Kirchner ChemPhysChem, 2023, e202300470; „Solvent Dependency of Catalyst-Substrate Aggregation Through π-π Stacking in Photoredox Catalysis”
DOI: 10.1002/cphc.202300470
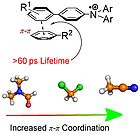
31. S, Fischer, T-T. H. Nguyen, A. Ratzenboeck, H. M. L. Davies, O. Reiser, Org. Lett., 2023, 25, 4411-4415; „Stereoselective Synthesis of Highly Functionalized Cyclohexenes via Strong-Acid-Mediated Endocyclic C-C Bond Cleavage of Monocyclopropanated Cyclopentadiens”
DOI: 10.1021/acs.orglett.3c00935
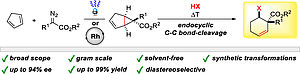
30. A. M. Arnold, P. Dullinger, A. Biswas, C. Jandl, D. Horinek, T. Gulder, Nat. Commun, 2023, 14, 813; „Enzyme-like polyene cyclizations catalyzed by dynamic, self-assembled, supramolecular fluoro alcohol-amine clusters”
DOI: 10.1038/s41467-023-36157-0
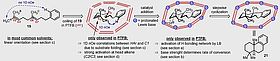
29. W. Stockerl, R. M. Gschwind, Chem. Commun, 2023, 59, 1325-1328; „Photo enhancement reveals (E,Z) and (Z,Z) configurations as additional intermediates in iminium ion catalysis”
DOI: 10.1039/D2CC05976B
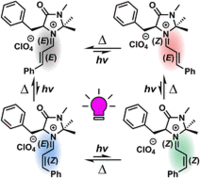
28. Y.-M. Tian, E. Hofmann, W. Silva, D. Touraud, R. M. Gschwind, W. Kunz, B. König, Angew. Chem. Int, Ed, 2023, 62, e202218775; „Enforced Electronic-Donor Complex formation in Water for Photochemical Cross-Coupling”
DOI: 10.1002/anie.202218775
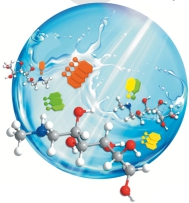
27. S. M. Tiefenthaler, N. Korber, Z. Anorg. Allg. Chem., 2023, 649, e202200286; „[K([2.2.2]-crypt)]K[Pt3(μ2-CO)3(PPh3)3]∙3 NH3 – A New Chini-Type Platinum Carbonyl Complex”
DOI: 10.1002/zaac.202200286
2022
26. J. Bauer, Organometallics, 2022, 41, 321-327; „Influence of Amino Functions on the Coordination Ability of Silyl Ethers and Disiloxanes”
DOI: 10.1021/acs.organomet.1c00663
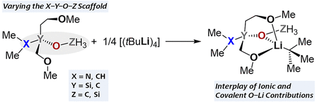
25. S. M. Tiefenthaler, F. Kleemiss, N. Korber, Z. Anorg. Allg. Chem., 2022, 648, e202100378; „[A([18]crown-6)]2[Pt(CO)3] ⋅ 10 NH3 (A=K, Rb) – A crystal structure containing the long postulated [Pt(CO)3]2−”
DOI: 10.1002/zaac.202100378
24. S. Yakubov, W. J. Stockel, X. Tian, A. Shahin, M. J. P. Mandigma, R. M. Gschwind, J. P. Barham, Chem., Sci., 2022, 13, 14041-14051; „Benzoates as photosensitization catalysts and auxiliaries in efficient, practical, light-powered direct C(sp3)–H fluorinations”
DOI: 10.1039/D2SC05735B
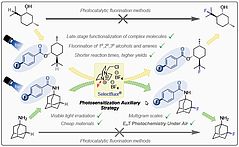
23. A. Falk, J. O. Bauer, Inorg. Chem. 2022, 61, 15576–15588; „Structural and Electronic Effects on Phosphine Chalcogenide Stabilized Silicon Centers in Four-Membered Heterocyclic Cations”
DOI: 10.1021/acs.inorgchem.2c02360

22. F. Babawale, K. Murugesan, R. Narobe, B. König, Org. Lett. 2022, 24, 4793–4797; „Synthesis of Unnatural α-Amino Acid Derivatives via Photoredox Activation of Inert C(sp3)–H Bonds”
DOI: 10.1021/acs.orglett.2c01822
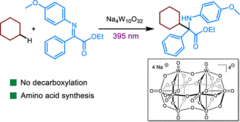
21. J. Gramüller, P. Dullinger, D. Horinek, R. M. Gschwind, Chem. Sci., 2022, 13, 14366-14372; „Bidentate Substrate Binding in Brønsted Acid Catalysis: Structural Space, Hydrogen Bonding and Dimerization”
DOI: 10.1039/D2SC05076E
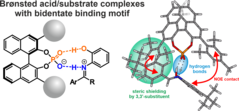
20. S. Wortmann, R. J. Kutta, P. Nuernberger, Front. Chem. 2022, 10:983342; „Monitoring the photochemistry of a formazan over 15 orders of magnitude in time”
DOI: 10.3389/fchem.2022.983342
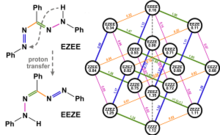
19. S. Yakubov, W. J. Stockerl, X. Tian, A. Shahin, M. J. P. Mandigma, R. M. Gschwind, J. P. Barham, Chem. Sci., 2022, 13, 14041-14051; „Benzoates as photosensitization catalysts and auxiliaries in efficient, practical, light-powered direct C(sp3)–H fluorinations”
DOI: 10.1039/D2SC05735B

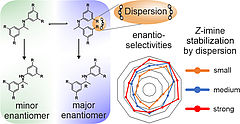
18. J. Gramüller, M. Franta, R. M. Gschwind, J. Am. Chem. Soc., 2022, 144, 19861-19871; „Tilting the Balance: London Dispersion Systematically Enhances Enantioselectivities in Brønsted Acid Catalyzed Transfer Hydrogenation of Imines”
DOI: 10.1021/jacs.2c07563
17. T. E. Schirmer, B. König, J. Am. Chem. Soc., 2022, 144, 19207-19218; „Ion-Pairing Catalysis in Stereoselective, Light-Induced Transformations”
DOI: 10.1021/jacs.2c04759
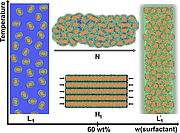
16. P. Denk, A. El Maangar, S. Prévost, W. Silva, R. M. Gschwind, T. Zemb, W. Kunz, J. Colloid Interface Science, 2022, 621, 470-488; „Cloud point, auto-coacervation, and nematic ordering of micelles formed by etlene oxide containing carboxylate surfactants”
DOI: 10.1016/j.jcis.2022.04.046
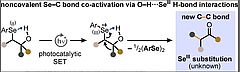
15. A. K. Dutta, S. Park, C. Allacher, A. Abramov, P. Dullinger, K. Kuzmanoska, D. Fritsch, P. Hitzfeld, D. Horinek, J. Rehbein, P. Nuernberger, R. M. Gschwind, A. Breder, Angew. Chem. Int. Ed., 2022, 61, e202208611; „Hydrogen bond-modulated nucleofugality of SeIII species to enable photoredox catalytic semipinacol manifolds”
DOI:10.1002/anie.202208611
14. K. Kristinaityte, A. Mames, M. Pietrzak, F. F. Westermair, W. Silva, R. M. Gschwind, T. Ratajczyk, M. Urba´nczyk, J. Am. Chem. Soc., 2022, 144, 13938-13945; „Deeper insight into photopolymerization: The Synergy of Time-Resolved Non-Uniform Sampling and Diffusion NMR”
DOI: 10.1021/jacs.2c05944
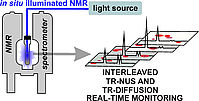
13. J. A. Kelly, V. Streitferdt, M. Dimitrova, F. F. Westermair, R. M. Gschwind, R. Berger, R. Wolf, J. Am. Chem. Soc., 2022, 144, 20434-20431; „Transition-Metal-Stabilised Heavy Tetraphospholide Anions”
DOI: 10.1021/jacs.2c08754
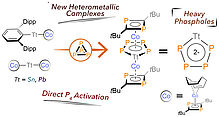
12. R. Eckl, S. Fischer, C. M. Sonnleitner, D. Schmidhuber, J. Rehbein, O. Reiser, ACS Org. Inorg. Au 2022, 2, 169-174; „Stereoselective Synthesis of Biologically Relevant Tetrahydropyridines and Dihydro-2H-pyrans via Ring-Expansion of Monocyclopropanated Heterocycles”
DOI: 10.1021/acsorginorgau.1c00042
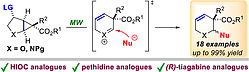
11. M. Zabka, R.M. Gschwind, Eur. J. Org. Chem. 2022, 2022, 26, e202200048; „Substrate Photoswitching for Rate Enhancement of an Organocatalytic Cyclization Reaction”
DOI: 10.1002/ejoc.202200048
![]()
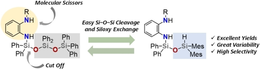
10. T. Götz, A. Falk, J. O. Bauer, Chem. Eur. J. 2022, 28, e202103531; „Molecular Scissors for Tailor-Made Modification of Siloxane Scaffolds”
DOI: 10.1002/chem.202103531
9. N. Fontana, N. A. Espinosa-Jalapa, M. Seidla, J. O. Bauer, Chem. Commun., 2022, 58, 2144-2147; „Hidden silylium-type reactivity of a siloxane-based phosphonium–hydroborate ion pair”
DOI: 10.1039/D1CC07016A

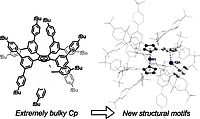
8. G. Hierlmeier, R. Wolf, Organometallics, 2022, 41, 776-784; „Bulking up CpBIG: A Penta-Terphenyl Cyclopentadienyl Ligand”
DOI: 10.1021/acs.organomet.2c00009
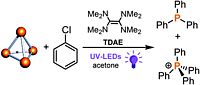
7. M. Till, V. Streitferdt, D. J. Scott, M. Mende, R.M. Gschwind, R. Wolf, Chem. Commun., 2022, 58, 1100-1103; „Photochemical transformation of chlorobenzenes and white phosphorus into arylphosphines and phosphonium salts”
DOI: 10.1039/d1cc05691c
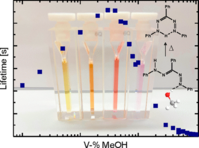
6. S. Wortmann, S. Schloeglmann, P. Nuernberger, J. Org. Chem. 2022, 87, 1745-1755; „Sensitivity of Isomerization Kinetics of 1,3,5-Triphenylformazan on Cosolvents Added to Toluene”
DOI: 10.1021/acs.joc.1c01928
2021
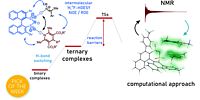
5. M. Zabka, R.M. Gschwind, Chem. Sci. 2021, 12, 15263-15272; "Ternary Complexes of Chiral Disulfonimides in Transfer-Hydrogenation of Imines: The Relevance of Late Intermediates in Ion Pair Catalysis"
DOI: 10.1039/D1SC03724B
4. M.J. Margeson, F. Seeberger, J. A. Kelly, J. Leitl, P. Coburger, R. Szlosek, C. Müller, R Wolf, ChemCatChem 2021, 13, 3761-3764; „Expedient Hydrofunctionalisation of Carbonyls and Imines Initiated by Phosphacyclohexadienyl Anions”
DOI: 10.1002/cctc.202100651
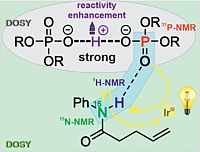
3. S. Karbalaei Khani, B. Geissler, E. Engelage, P. Nuernberger, C. Hättig, Phys. Chem. Chem. Phys. 2021, 23, 7480–7494; „Tracing absorption and emission characteristics of halogen-bonded ion pairs involving halogenated imidazolium species”
DOI: 10.1039/d1cp00009h
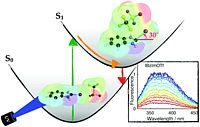
2. N. Berg, S. Bergwinkl, P. Nuernberger, D. Horinek, R.M. Gschwind, J. Am. Chem. Soc., 2021, 143, 724-735; „Extended Hydrogen Bond Networks for Effective Proton-Coupled Electron Transfer (PCET) Reactions: The Unexpected Role of Thiophenol and Its Acidic Channel in Photocatalytic Hydroamidations”
DOI: 10.1021/jacs.0c08673
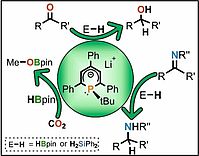
1. J.. Kelly, J. Gramüller, R. Wolf, R.M. Gschwind, Dalton Trans., 2021, 50, 13985-13992; „Low-oxidation state cobalt-magnesium complexes: ion-pairing and reactivitys”
DOI: 10.1039/d1dt02621f

For publications from single PI's -> see homepage of the supervisor
2020
J. Leitl, A. R. Jupp, E. R. M. Habraken, V. Streitferdt, P. Coburger, R. M. Gschwind, C. Müller, J. C. Slootweg, R. Wolf, Chem. Eur. J. 2020, 26, 7788-780; “A phosphinine-derived 1-phospha-7-bora-norbornadiene: frustrated Lewis pair type activation toward triple bonds“
DOI: 10.1002/chem.202000266
2019
S. Wang, N. Lokesh, J. Hioe, R. Gschwind, B. König; Chem Sci. 2019, 10, 4580-4587; “Photoinitiated Carbonyl‐Metathesis: Deoxygenative Reductive Olefination of Aromatic Aldehydes via Photoredox Catalys“
DOI: 10.1039/C9SC00711C
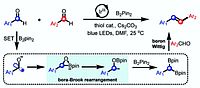
D. Petzold, P. Nitschke, F. Brandl, V. Scheidler, B. Dick, R. M. Gschwind, B. König; Chem. Eur. J. 2019, 25, 361-366; “Visible Light Mediated Liberation and in situ Conversion of Fluorophosgene”
DOI: 10.1002/chem.201804603
A. C. Kneuttinger, K. Straub, P. Bittner, N. Simeth, A. Bruckmann, F. Busch, C. Rajendran, E. Hupfeld, V. H. Wysocki, D. Horinek, B. König, R. Merkl, R. Sterner; Cell Chem. Biol. 2019, 26, 1501-1514; ”Light-Regulation of Enzyme Allostery through Photo-Responsive Unnatural Amino Acids”
DOI: 10.1016/j.chembiol.2019.08.006
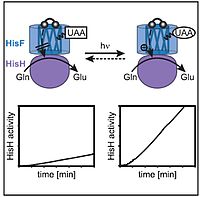
F. Hastreiter, C. Lorenz, J. Hioe, S. Gärtner, L. Nanjundappa, N. Korber, R. M. Gschwind, Angew. Chem. Int. Ed., 2019, 58, 3133-3137; ”Elusive Zintl Ions [µ HSi4]3− and [Si5]2− in Liquid Ammonia: Protonation States, Sites, and Bonding Situation by NMR and Theory”
DOI: 10.1002/ange.201812955
T. Pongratz, P. Kibies, L. Eberlein, N. Tielker, C. Hölzl, S. Imoto, M. Beck Erlach, S. Kurrmann, P. H. Schummel, M. Hofmann, O. Reiser, R. Winter, W. Kremer, H. R. Kalbitzer, D. Marx, D. Horinek, S. M. Kast Biophys. Chem. 2019, 106258; ”Pressure-dependent electronic structure calculations using integral equation-based solvation models”
DOI: 10.1016/j.bpc.2019.106258

S. Kerres, S. Malcherek, J. Rehbein, O. Reiser, Adv. Synth. Catal. 2019, 361, 1400-1407; ”Visible Light Mediated Synthesis of enantiopure γ-Cyclobutane Amino and 3-(Aminomethyl)-5-phenylpentanoic Acids”
DOI: 10.1002/adsc.201801413
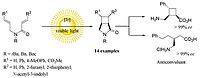
U. Lennert, P.B. Arockiam, V. Streitferdt, D.J. Scott, C. Rödl, R.M. Gschwind, R. Wolf, Nat. Cat. 2019, 2, 1101-1106; “Direct Catalytic Transformation of White Phosphorus into Aryl Phosphines and Phosphonium Salts“
DOI: 10.1038/s41929-019-0378-4
J. Leitl
J. Leitl, M. Marquardt, P. Coburger, D. J. Scott, V. Streitferdt, R. M. Gschwind, C. Müller, R. Wolf, Angew. Chem. Int. Ed. 2019, 58, 15407-15411; “Facile C=O Bond Splitting of Carbon Dioxide Induced by Metal‑Ligand Cooperativity in a Phosphinine Iron(0) Complex“
DOI: 10.1002 / anie.201909240
2018
L. Marzo, S. K. Pagire, O. Reiser, B. König, Angew. Chem. Int. Ed. 2018, 57, 10034 – 10072; ”Visible-Light Photocatalysis: Does it make a difference in Organic Synthesis?”
DOI; 10.1002/anie.201709766
C. Lorenz, F. Hastreiter, J. Hioe, L. Nanjundappa, S. Gärtner, N. Korber, R.M. Gschwind, Angew. Chem. Int. Ed. 2018, 57, 12956-12960; “Structure of [HSi₉]³⁻ in Solid State and Its Unexpectedly High Dynamics in Solution”
DOI: 10.1002/anie.201807080
R.J. Wilson, F. Hastreiter, K. Reiter, P. Büschelberger, R. Wolf, R.M. Gschwind, F. Weigend, S. Dehnen, Angew. Chem. Int. Ed. 2018, 57, 15359-15363; “[Co@Sn6Sb6]3−: An Off‐Center Endohedral 12‐Vertex Cluster”
DOI: 10.1002/anie.201807180
T. Föll, J. Rehbein, O. Reiser, Org. Lett. 2018, 20, 5794-5798; “Ir(ppy)3-Catalyzed, Visible-Light-Mediated Reaction of α-Chloro Cinnamates with Enol Acetates: An Apparent Halogen Paradox”
DOI: 10.1021/acs.orglett.8b02484
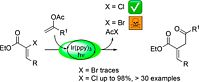
A. Hossain, A. Vidyasagar, C. Eichinger, C. Lankes, J. Phan, J. Rehbein, O. Reiser, Angew. Chem. Int. Ed. Engl. 2018, 57, 8288-8292; ”Visible-Light-Accelerated Copper(II) Catalyzed Regio- and Chemoselective Oxo-Azidation of Vinyl Arenes”
DOI: 10.1002/anie.201801678
A. Das, M. Maity, S. Malcherek, B. König, J. Rehbein, Beilstein J. Org. Chem. 2018, 14, 2520–2528; ”Synthesis of aryl sulfides via radical–radical cross coupling of electron-rich arenes using visible light photoredox catalysis”
DOI: 10.3762/bjoc.14.228
2017
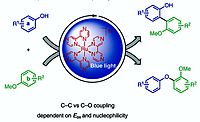
A. Eisenhofer, J. Hioe, R. Gschwind, B. König, Eur. J. Org. Chem. 2017, 2194–2204; ”Photocatalytic Phenol-Arene C-C and C-O Cross-Dehydrogenative Coupling”
DOI: 10.1002/ejoc.201700211
2016
T. Hering, B. Mühldorf, R. Wolf, B, König, Angew. Chem. Int. Ed. 2016, 55, 5342-5345; "Halogenase-Inspired Oxidative Chlorination Using Flavin Photocatalysis"
DOI: 10.1002/anie.201600783
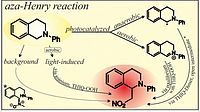
H. Bartling, A. Eisenhofer, B. König, R.M. Gschwind, J. Am. Chem. Soc. 2016, 138, 11860-11871; “The Photocatalyzed Aza-Henry Reaction of N-Aryltetrahydroisoquinolines: Comprehensive Mechanism, H•- versus H+-Abstraction, and Background Reactions”
DOI: 10.1021/jacs.6b06658
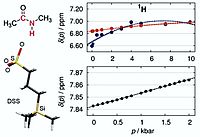
R. Frach, P. Kibies, S. Böttcher, T. Pongratz, S. Strohfeldt, S. Kurrmann, J. Koehler, M. Hofmann, W. Kremer, H. R. Kalbitzer, O. Reiser, D. Horinek, S. M. Kast, Angew. Chem. Int. Ed. 2016, 55, 8757; „The Chemical Shift Baseline for High-Pressure NMR Spectroscopy of Proteins“
DOI: 10.1002/anie.201602054.
H. Jangra, M.H. Haindl, F. Achrainer, J. Hioe, R.M. Gschwind, H. Zipse, Chem. Eur. J. 2016, 22, 13328-13335; “Conformational Preferences in Small Peptide Models: The Relevance of cis/trans-Conformations”
DOI: 10.1002/chem.201601828
2015
F. Fendt, C. Koch, M. Neumeier, S. Gärtner, R.M. Gschwind, N. Korber, Chem. Eur. J. 2015, 21, 14539-14544; “Stability and Conversion of Tin Zintl Anions in Liquid Ammonia Investigated by NMR Spectroscopy”
DOI: 10.1002/chem.201501100
2014
S. Gärtner, T. Gärtner, R.M. Gschwind, N. Korber, Acta Cryst. 2014, E70, 555-558; „About the polymorphism [Li(C4H8O)3]I:crystal structures of trigonal and tetragonal polymorphs”
DOI: 10.1107/S160053681402529X
2013
J. Daďová, S. Kümmel, C. Feldmeier, J. Cibulkova, R. Pazout, J. Maixner, R.M. Gschwind, B. König, R. Cibulka, Chem. Eur. J. 2013, 19, 1066-1075; “Aggregation Effects in Visible-Light Flavin Photocatalysts: Synthesis, Structure, and Catalytic Activity of 10-Arylflavins”
DOI: 10.1002/chem.201202488
M. Neumeier, F. Fendt, S. Gärtner, C. Koch, T. Gärtner, N. Korber, R.M. Gschwind, Angew. Chem. Int. Ed. 2013, 52, 4483-4486; “Detection of the Elusive Highly Charged Zintl Ions Si4(4-) and Sn4(4-) in Liquid Ammonia by NMR Spectroscopy”
DOI: 10.1002/anie.201209578
2010
T. Thaler, B. Haag, A. Gavryushin, K. Schober, E. Hartmann, R.M. Gschwind, H. Zipse, P. Mayer, P. Knochel, Nature Chemistry 2010, 2, 125-130; “Highly diastereoselective Csp3–Csp2 Nis cross-coupling with 1,2-, 1,3- and 1,4-substituted cyclokylzinc compounds“
DOI: 10.1038/NCHEM.505
2009
M. Schmid, M. Fleischmann, V. D´Elia, O. Reiser, W. Gronwald, R.M. Gschwind, ChemBioChem, 2009, 10, 440–444; „RDCs in Short Peptidic Foldamers: Combined Analyses of Backbone and Side Chain Conformations and Evaluation of Structure Coordinates of Rigid Unnatural Amino Acids”
DOI: 10.1002/cbic.200800736