News of the Department
Property tailored lignin extraction using a biodiverse GVL-based aldehyde-assisted organosolv process towards use of lignin in specific applications
by Moritz Schweiger, Eva Mueller, Thomas Lang, Didier Touraud, Werner Kunz
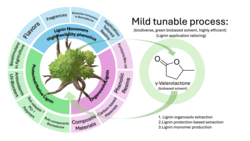 |  |
The poster "property tailored lignin extraction using a biodiverse GVL-based aldehyde-assisted organosolv process towards use of lignin in specific applications" highlights a crucial aspect of current state of the art lignin research. The structural complexity and inconsistency of lignin hinder the efficient separation from biomass and acts as a barrier to ist efficient valorization. Using γ-valerolactone (GVL), a sustainable solvent, we have developed a mild process that efficiently fractionates diverse types of biomass into high-purity and high-yield lignin, cellulose, and hemicellulose. We can adjust the biomass source and the process parameters to effectively tailor the properties of the resulting lignin, particularly its molecular weight, hydroxy group content, ether content, and subunit composition. This enables the targeted production of lignin products with optimized properties for use in specific commercial applications. The poster presents a versatile and biodiverse approach to lignin valorization, creating a link between efficient, green biomass fractionation, property-specific lignin production, and the subsequent potential for manufacturing high-value chemicals.
For his contribution at Faraday Discussion - Frontiers in physical chemistry for lignin valorisation in London, Moritz Schweiger obtained the poster prize for Green Chemistry/Sustainability.

Concept and Applications of Metal-Based COMPLET Ionic Liquids
by Selina Reigl, Julia Loposko, Patrick Denk, Sebastian Koltzenburg, Matthias Kellermeier and Werner Kunz
|
|
|
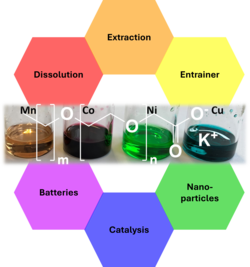
The poster describes the synthesis and properties of novel COMPLET-ILs (COncept of Melting Point Lowering due to EThoxylation) based on alkyl ether carboxylates. Their combination with different cations provides access to cost-efficient, non-toxic ionic liquids with low melting points and, if desired, surfactant-like behavior. Key properties such as conductivity, viscosity, and coordination behavior were characterized. The focus was placed on potential applications including the recycling and separation of metal salts, the separation of aromatics and aliphatics, catalytic reactions, electrochemical processes, energy storage, and the synthesis of nanoparticles.
For her contribution at ILMAT 2025 - 8th international conference for ionic liquid based materials in Rome, Selina Reigl obtained the prize for the best poster.
 |  |
Innovative Approaches to Industrial Extraction – Insights from Prof. Jochen Strube

As part of our chair seminar series, we were pleased to host Prof. Strube from Clausthal University of Technology. His talk provided valuable engineering insights into the current challenges and innovations in industrial extraction processes.
Prof. Strube emphasized the importance of using modern, up-to-date methods for the extraction of natural products—a market experiencing significant growth. He presented innovative approaches based on both thermodynamically and kinetically controlled extraction, underlining their potential for improving efficiency and reducing costs. Fluid dynamics in both small and large-scale reactors were discussed, with a particular focus on improving prediction accuracy. These insights can also support regulatory compliance, especially with respect to upcoming EU legislation aiming for greenhouse gas neutrality by 2050.
A key aspect of the lecture was the application of the digital twin concept using machine learning. This technology enables better process optimization and real-time adjustments, which are particularly important in scaling up from lab to industrial level.
While natural products are trending, industrial applications face different regulatory challenges. Supplements, for example, have fewer hurdles compared to medical products. However, many current industrial methods remain outdated, leaving significant room for improvement—especially in terms of energy savings and sustainability.
Prof. Strube concluded by highlighting the need for both imagination and technical competence in advancing industrial extraction methods to meet current and future demands.
Innovative Luminescence Technique Presented by Prof. Uday Maitra
In a fascinating lecture held on May 7th in the frame of our work group seminar, Professor Uday Maitra from the Indian Institute of Science (IISc), Department of Organic Chemistry in Bangalore, presented a novel method to enhance luminescence from lanthanides, offering promising applications in medical diagnostics and drug detection.
Prof. Maitra’s team has developed a technique based on self-assembly in cholate hydrogels, which significantly boosts the luminescence of lanthanides using specially designed sensitizers. This improved signal can be used to detect enzymes, polyphenols, and pharmaceutical compounds with remarkable speed and precision.
The method’s high sensitivity makes it particularly suited for applications in healthcare, including the rapid identification of multi-resistant bacteria or targeted drug screening. Researchers and students alike praised the approach for its scientific innovation and real-world potential.
The event was part of our ongoing seminar series aimed at showcasing cutting-edge research from around the globe.
Scientist Turned Entrepreneur: Dr. Thomas Buchecker Shares His Journey

May 21 –
Drawing from his dual experience in industry and entrepreneurship, Dr. Buchecker offered a candid look at the transition from academic research to the competitive world of business. He highlighted the contrasts between being employed and running one’s own company, noting that while employment offers stability and structured development, entrepreneurship allows for creative freedom and personal impact—though not without risk.
He also explained the differences between product-based businesses and consulting models, emphasizing that each path demands a unique mindset and skillset. Attendees praised the session for its honesty and practical relevance, especially for young scientists considering their future beyond academia.
Stefan Wolfrum Shares Insights on Transitioning from Academia to Industry

Yesterday, former doctoral student of our chair, Stefan Wolfrum, now working at Wacker Chemie, gave a talk as part of our seminar series. He shared valuable experiences, insights, and challenges he encountered when transitioning from academia to industry.
His presentation provided an in-depth perspective on the differences in research environments, expectations, and problem-solving approaches between the two fields. The discussion was particularly relevant for young researchers considering a career in industry, offering practical advice on navigating this transition successfully.
Lukas Zeininger Presents Insights on Trigger-Responsive Nanoemulsions

On February 5th, Lukas Zeininger, holder of an Emmy Noether Professorship at the Max Planck Institute in Potsdam, delivered a well-attended in-person lecture as part of our chair’s seminar series. His talk provided valuable insights into the mechanisms and practical applications of trigger-responsive "Janus"-nanoemulsions.
These innovative materials possess the ability to autonomously react to environmental changes, making them highly promising for applications in drug delivery, catalysis and sensoring. Zeininger’s research has significantly contributed to harnessing the adaptive capabilities of droplets for practical use.
His presentation resonated strongly across the faculty, attracting the interest of numerous researchers, including the Dean and Vice Dean, who were also in attendance.
Exploring Complex Colloidal Systems: Kevin Roger’s Insights at our Workgroup Seminar
Recently, Kevin Roger, a young scientist and researcher at CNRS (Chargé de Recherche CNRS), delivered an insightful presentation in the frame of our weekly work group seminar. His research focuses on understanding how complex colloidal systems structure themselves under non-equilibrium conditions, with particular emphasis on drying, emulsification, and precipitation.
During his talk, Roger highlighted key findings on drying, demonstrating that humidity plays a surprisingly minor role in water evaporation from colloidal systems. Instead, changes in water activity and permeability near the air-liquid interface drive the process. He further gave amazing insight into nanopreipitation phenomena and the Ouzo-effect.
Roger’s presentation underscored the significance of colloidal science in both fundamental research and industrial applications, sparking engaging discussions among attendees.
Inspiring Perspectives: John C. Warner on the Future of Green Chemistry (#greenchemistry, #biomimicry, #John Warner)

Last week, our work group seminar had the privilege of hosting a fascinating presentation by John C. Warner, a pioneering figure in green chemistry. Known for co-founding the field, Warner has built an impressive career spanning industry, academia, and entrepreneurship.
During his talk, he shared insights into his professional journey, from his early research at Polaroid Corporation to his academic roles at the University of Massachusetts Boston and Lowell. He also discussed his work as co-founder and President of the Warner-Babcock Institute for Green Chemistry and Beyond Benign, organizations dedicated to advancing sustainable chemistry. His contributions have been widely recognized, including receiving the 2014 Perkin Medal, one of the highest honors in American industrial chemistry.
The core message of his presentation was clear and compelling: as chemists, we have a responsibility to ensure that future generations are equipped with the knowledge and skills to develop truly sustainable technologies. Warner’s vision is not just about innovation but about transforming the way chemistry is taught and practiced, making sustainability an integral part of the discipline.
His inspiring talk sparked valuable discussions and reinforced the importance of green chemistry in shaping a more sustainable future.
Biliquid oil-in-water nanofoams and spontaneous emulsification obtained with a surfactant resistant to curvature changes

Hypothesis
Due to its huge polar headgroup, octaoxyethylene octyl ether carboxylic acid (C8E8CH2COOH= Akypo LF2™) is supposed not to be able to change its curvature sufficiently to form bicontinuous microemulsions. Instead, upon adding an oil to the binary water – surfactant system, excess oil could be squeezed out or a biliquid foam could form.
Experiments
An auto-dilution setup was used to record small-angle X-ray scattering data along six dilution lines in the newly established phase diagram of the ternary system 2-ethylhexanol – C8E8CH2COOH – water.
Results
Evaluation of the data in combination with the recorded phase diagram revealed that the ternary microemulsions with aslightly amphiphilic oil indeed do not show a classical structural inversion via a bicontinuous structure with increasing oil content, but instead the sequence: O/W micelles – O/W biliquid nanofoam – molecular co-solubilization in the oil phase. The biliquid nanofoam structure with 102–104 oil molecules enclosed by locally flat layers of interdigitated hydrated headgroups exists in the middle of the phase diagram. We may speculate that this phase can be used as a multitude of nanocontainers, e.g., for chemical reactions in an aqueous environment, but with negligible water chemical potential. In the vicinity of the critical point, spontaneous formation of stable mesoscale droplets (an “Onuki-like” structure, as known with antagonistic salts) is detected in a region showing a pronounced Tyndall effect (
Nanodots of Transition Metal Sulfides, Carbonates, and Oxides Obtained Through Spontaneous Co-Precipitation with Silica

The controlled formation and stabilization of nanoparticles is of fundamental relevance for materials science and key to many modern technologies. Common synthetic strategies to arrest growth at small sizes and prevent undesired particle agglomeration often rely on the use of organic additives and require non-aqueous media and/or high temperatures, all of which appear critical with respect to production costs, safety and sustainability. In the present work, we show that parti- cles of various transition metal carbonates and sulfides with sizes of only few nanometers can read- ily be obtained through a simple one-pot process in water and at ambient conditions. To this end, solutions of soluble salts of metal cations (e.g., chlorides) and the respective anions (e.g., sodium carbonate or sulfide) are mixed in the presence of different amounts of sodium silicate at elevated pH levels. Upon mixing, metal carbonate/sulfide particles nucleate and their subsequent growth causes a sensible decrease of pH in the vicinity. Dissolved silicate species respond to this local acid- ification by condensation reactions, which eventually lead to the formation of amorphous silica lay- ers that encapsulate the metal carbonate/sulfide cores and thus effectively inhibit any further growth. The as-obtained carbonate nanodots can readily be converted into the corresponding metal oxides by secondary thermal treatment, during which their nanometric size is maintained. Although the described method clearly requires optimization towards actual applications, the results of this study highlight the potential of bottom-up self-assembly for the synthesis of functional nanoparticles at mild conditions
(https://doi.org/10.3390/nano14242054).Renowned Scientist Vince Craig Presents Latest Research Findings on Specific Ion Effects

As part of our weekly staff seminar, we had the privilege of welcoming a distinguished guest: Vince Craig, a renowned scientist from the Australian National University in Canberra, whose career has been largely dedicated to the study of specific ion effects.
Craig, internationally acclaimed for his work in this field, provided a fascinating insight into his latest research findings. His presentation focused on the question of how the Hofmeister series and the lyotropic series can be maintained in various solvents. These two concepts, which play a central role in chemistry and biophysics, were illuminated in a new light and illustrated with compelling practical examples.
His lecture opened exciting new perspectives for the application of these insights in both fundamental and applied research. The participants were deeply impressed and expressed their gratitude to Craig for the inspiring ideas he shared during his talk.
With this event, our seminar continues its tradition of hosting high-caliber guests and fostering interdisciplinary exchange.
Presentation at our Workgroup Seminar: Water-in-Water Emulsions

On wednesday July 18th, Dr. Jordi Esquena from the Institute of Advanced Chemistry of Catalonia (IQAC), part of the Consejo Superior de Investigaciones Científicas (CSIC), delivered a fascinating lecture on the topic of water-in-water emulsions during our weekly workgroup seminar. His research focuses on the complex mechanisms of liquid-liquid phase separations in aqueous systems and provides new insights into the potential applications of such systems.
Liquid/Liquid Phase Separations in Aqueous Environments
Dr. Esquena explained the fundamentals of phase separations in aqueous environments, which can be induced by various mechanisms. He particularly highlighted the role of salts and their ability to cause phase separations through the so-called "salting-out" effect. These phenomena occur when the ions of the salts alter the water structure, displacing certain macromolecules from the solution and causing them to form a separate phase.
Another focus of the presentation was the interactions between polyelectrolytes and polymers. These interactions can also induce phase separations, with the specific combination of polyelectrolytes and polymers being crucial for the stability and properties of the resulting emulsion. These systems offer unique opportunities to stabilize sensitive biomolecules, such as enzymes, in aqueous environments.
Stabilization of Enzymes and Controlled Release Systems
One particularly interesting application of these phase separations is the stabilization of enzymes, such as lactase. Dr. Esquena demonstrated how selecting appropriate polymers and their interactions with water can achieve controlled and retarded release of these enzymes. Such systems are particularly valuable as they protect the enzyme from the harsh conditions of digestive fluids, thereby increasing the effectiveness and longevity of enzyme preparations.
Our Group Outing to the geodetic fundamental station Wettzell

On August 1, 2024, we, the solution chemistry group, took a group outing to Bad Kötzting. In beautiful weather, we began our ascent on the Planetenweg, which features informational pillars about the solar system leading up to Wettzell. Upon arrival, we visited the geodetic fundamental station and received an interesting and informative tour from Dr. Thomas Klügel. After descending via a shaded hiking trail, we returned to Bad Kötzting, where we ended the day with a relaxing visit to a beer garden.
Prof. Michael Sternad Presents on Electrochemical Energy Storage

Prof. Michael Sternad of TH-Deggendorf recently delivered an insightful lecture on electrochemical energy storage as part of the Chair Seminar series. The presentation highlighted the work being done by his research group at the Technology Campus Plattling, providing a comprehensive overview of current technologies and future advancements in the field.
The lecture began with a brief introduction to the research group led by Prof. Sternad at the Technology Campus in Plattling. This team is at the forefront of innovation, dedicated to advancing the science of electrochemical energy storage through rigorous research and development.
Prof. Sternad then provided an overview of the fundamental components and functions of batteries and fuel cells. These energy storage devices consist of electrodes, separator, electrolyte and various -but important- electrolyte additives. The lecture emphasized the critical role of electrolytes and additives in enhancing the performance and longevity of these systems.
Delving deeper, Prof. Sternad explained the specific functions and requirements of electrolytes and their additives. The right combination of electrolytes and additives is essential for optimizing the efficiency and durability of electrochemical cells.
The discussion then shifted to secondary lithium-ion batteries, highlighting their advantages and unique features.
Concluding the lecture, Prof. Sternad provided a glimpse into future technologies currently under development. His team is exploring novel concepts of Li-metal batteries (“anode-free” design) and advanced active materials for electrodes, innovative coatings for traditional electrodes, and new electrolytes capable of supporting higher cell voltages. These advancements aim to further enhance the performance and safety of electrochemical energy storage systems.
Prof. Sternad's lecture underscored the significant progress being made in the field of electrochemical energy storage and the exciting potential of future innovations. The work being done at the Plattling Technology Campus promises to contribute substantially to the advancement of energy storage technologies.
Our weekly group seminar: Innovative Biopolymer Research Highlighted by Young Scientist from University of Kraków

Ewelina Jamroz from the University of Kraków, Poland. As a promising young scientist, Prof. Jamroz delivered an insightful presentation on furcellaran, a biopolymer derived from red algae.
Prof. Jamroz highlighted the multiple potential applications of furcellaran, emphasizing its use in creating biodegradable films and coatings. This innovative research could pave the way for more sustainable materials in various industries, aligning with the growing demand for environmentally friendly solutions.
Her presentation underscored the importance of exploring natural polymers to address ecological challenges, sparking lively discussions and interest among attendees. The work group seminar concluded on an optimistic note, inspired by the promising advancements in biopolymer research led by young scientists like Prof. Jamroz.
In Folge 10 des UR-Podcasts Gasthörer: Nachhaltige Chemie mit Professor Dr. Werner Kunz
Chemie ist nicht immer böse! Diese Botschaft ist das Anliegen von Professor Dr. Werner Kunz, Lehrstuhl für Physikalische Chemie an der Universität Regensburg. Der Wissenschaftler und sein Team forschen seit Jahrzehnten an umweltfreundlichen Lösungen für bestehende Produkte: Ob Körperpflege oder Haushaltsreiniger, Medikamente oder Düngemittel – für all diese Produkte gibt es Varianten, die genauso gut funktionieren, aber gleichzeitig nachhaltig und nicht giftig sind.

Prof. Dr. Werner Kunz. Fotos und Podcast: Katharina Herkommer / UR
Kunz und sein Team haben in den vergangenen Jahren unter anderem einen wirksamen Graffiti-Entferner entwickelt, den man sogar trinken könnte, oder auch ein umweltfreundliches Shampoo, das die Haare auf ganz besondere Weise pflegt. Wie sich diese Forschung auf dem Gebiet der Lösungsmittelchemie gestaltet und was es mit Erfindungen und Patenten auf sich hat, erklärt er in Folge 10 des UR-Podcasts Gasthörer.

Chemielaborantin Theresa Ferstl, Mitarbeiterin am Lehrstuhl Kunz, im Labor an der Putzmaschine.
Vorlesung Colloids II im Masterstudium Chemie >see here
Seminar zum Praktikum Formulierung im Masterstudium Chemie bzw. Wirtschaft/Chemie >see here
Vorlesung Technische Chemie in den Bachelorstudiengängen Chemie und Chemie/Wirtschaft >see here
Bachelorarbeitsthemen 2021 >see here
"Interessierte an den jeweiligen Themen können für nähere Infos Kontakt zu den jeweiligen Betreuern aufnehmen, die zu den Themen angegeben sind (unter N.N@chemie.uni-r.de). Die Anmeldung muss dann offiziell über das Lehrstuhlsekretariat erfolgen. Dies kann geschehen, sobald Sie dazu die offizielle Zulassung durch das Prüfungsamt erhalten haben."
Liebe Studierende,
bitte laden Sie den Zoom-Link und die entsprechenden Unterlagen aus GRIPS von der Veranstaltung herunter:
Elektrochemie/Transporteigenschaften 3. Sem., 20/21
Dear Students,
those of you, who are interested in the lecture FORMULATION, please go in GRIPS to my "Lecture Formulation".
In the announcements, you can find further information and download the ZOOM-Link.
Protokoll zur jährlichen Unterweisung 2020 :>hier
Hinweise und Informationen für Prüfer und Aufsichten zum Ablauf von schriftlichen Prüfungen im Sommersemester 2020
SHK-Job zu vergeben (September bis November 2020):638 Euro pro Monat für 3 Monate
Im Rahmen eines vom bayerischen Umweltministeriums geförderten Projektes zur Wiederaufarbeitung von PVC und des damit verbundenen Recyclings wertvoller Metalle ist ab 1. September eine SHK-Stelle am Lehrstuhl von Prof. Kunz zu vergeben.
Die studentische Hilfskraft soll im Rahmen des Projektes „Chlor-Plattform“ folgende Tätigkeiten an der Universität Regensburg durchführen.
· Fällungsreaktionen und Filtrationen
- Zur Entfernung der kohlenstoffhaltigen Schlacke oder sonstigen Verunreinigungen aus den Metallkonzentraten sollen verschiedene Fällungsreagenzien verwendet werden.
· Flüssig-Flüssig-/Fest-Flüssig-Extraktionen
- Die Metalle sollen durch Extraktionen von Verunreinigungen befreit werden. Hierzu werden verschiedenste klassische Lösungsmittel verwendet. Ebenfalls soll untersucht werden, ob weitere Metalle z.B. Pb aus den PVC-Koksen aufgrund der Schadstoffproblematik zurückgewonnen werden kann.
· Fraktionierende Destillation
- Störstoffe in den Metallkonzentraten sollen vor oder nach der Extraktion entfernt werden.
Die Versuche sollten eigenständig nach Anleitung durchgeführt werden. Ebenfalls ist eine gute Dokumentation der Versuchsdurchführung sowie Ergebnisbericht wichtig.
Computational Fluid Phase Thermodynamics (53185)
ab 5. Okt. 2020
Professor Dr. Andreas Klamt
(2 SWS, Anrechnung für Aufbaumodul II in
Kombination mit anderer Vorlesung möglich)
des Institutes für Physikalische und Theoretische Chemie für wissenschaftliche Mitarbeiter und Studenten nach der Bachelorarbeit Wintersemester 2020/21
Internal Meeting
Meeting schedule for regular seminar on Wednesday at 17:00 p.m.:
Link only visible after login

Older News (2021-2023)
The best surfactant ... is no surfactant

Today, industrial chemical reactions are usually made in organic solvents. Due to environmental concerns and the search for new, sustainable processes, there is intense research for reactions that can be made in aqueous solutions. Of course, the lack of solubility of hydrophobic reactants is the major obstacle. To overcome this problem, usually surfactants are added to water. The result is called “micellar catalysis”. In a series of publications in high-impact journals, an American colleague has convinced the community and also industrial companies that micelles are mandatory for optimised reactivity in aqueous systems. Even more, the reactions work best with the very specific surfactants developed in his group. Since we had some doubts, we repeated some of the reactions published with micellar catalysis, but this time without any surfactant, just by performing the chemical transfer in simple mixtures of water and a simple alcohol. And it turned out, the yield was even better, and the conditions were milder! The crucial parameter is not the confinement in micelles, it is simply a sufficient and proper solubility of the reactants. Having proven this, we can now proceed to significantly simplify chemical reactions of industrial relevance. The results are published in the high-impact journal (IF = 16,74) Chemical Engineering Journal (https://doi.org/10.1016/j.cej.2023.144599).
Preis für nachhaltige Produkte und Verfahren
 |  |
Auf der diesjährigen internationalen Konferenz Formula XI in Lille, Frankreich, wurde Prof. Kunz von der französischen chemischen Gesellschaft für seine jahrzehntelangen Beiträge zur Herstellung nachhaltigerer Produkte, besonders im Bereich Kosmetik und Reinigungsmittel und zur umweltfreundlicheren Produktion von Chemikalien mit dem Pierre-Fillet-Preis geehrt. Die Auszeichnung wird alle drei Jahre für Persönlichkeiten vergeben, die sich besonders um die verbesserte Formulierung von gebrauchsfertigen Produkten verdient gemacht haben. Nach dem Steinkopff-Preis 2019 der deutschen Kolloidgesellschaft ist dies bereits der zweite Preis für nachhaltige Chemie, den Prof. Kunz erhalten hat. Neben dieser Auszeichnung durfte Prof. Kunz im Rahmen eines 60-minütigen Hauptvortrages die wesentlichen Ergebnisse seiner Arbeitsgruppe in den letzten Jahren einem internationalen Publikum vorstellen. Dabei legte er besonderen Wert auf den Aspekt der Nachhaltigkeit der entwickelten Produkte und Verfahren, wobei nicht nur wissenschaftliche Aspekte (Umwelt, Energie, Toxizität) angesprochen wurden, sondern auch Performance, Preis, Kundenakzeptanz und die soziale Komponente. Gerade dieser ganzheitliche Ansatz bei der Umsetzung von nachhaltigen Ideen liegt Prof. Kunz besonders am Herzen.
A new strategy to dissolve long-chain surfactants in water at low temperatures
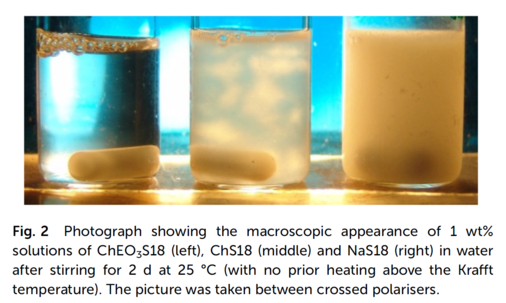
Surfactants find widespread use in daily life for cleaning purposes and form a vital part of many industrial formulations. So far, their application has been limited to amphiphilic structures with relatively short alkyl chains (typically up to C14 or C16) due to the poor solubility of longer-chain homologues under relevant conditions. Here we introduce a concept that eventually allows octadecyl sulfates and carboxylates to be effectively solubilised in water at room temperature. Through synthesis of alkoxylated derivatives of choline – an abundant molecule of natural origin – we have designed a new class of counterions preventing the precipitation of long-chain surfactants, as commonly observed with alkali ions or unmodified choline. The resulting amphiphilic systems show superior properties with respect to surface activity, which directly translates into enhanced cleaning performance in lab-based washing tests. Studies on the cytotoxicity and biodegradability of the alkoxylated choline derivatives highlight their potential for sustainable surfactant development. In the end, our approach could pave the way towards the use of hitherto unleveraged raw material resources in tailoured surfactant formulations for cleaning applications and beyond.
Physical-chemical and toxicological properties of osmolyte-based cationic surfactants and spontaneously formed low-toxic catanionic vesicles out of them
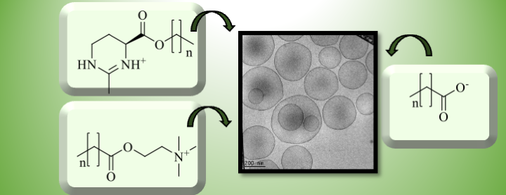
In the context of efficient drug delivery systems, catanionic vesicles offer several advantages, such as spontaneous formation and long-term stability. However, especially the cationic component of such vesicles is often toxic. Thus, the search for less toxic, biocompatible amphiphiles, while maintaining the desirable aggregation properties is crucial. In this work, we present cytotoxicity towards the human cell line Hela as well as biodegradability data of some cationic surfactants based on the natural osmolytes choline and ectoine. The synthesis, aggregation and solubility behaviour as well as the stability in water of these compounds is discussed. In order to induce the spontaneous formation of vesicles, several of these cationic surfactants are combined with choline carboxylates with varying chain length in different mixing ratios and characterised with cryo-TEM. Further, the cytotoxic effect of such novel catanionics is evaluated as a function of the cationic-anionic ratio and compared to that of classical combinations of sodium dodecylsulfate and dodecyltrimethylammonium bromide.
doi.org/10.1016/j.molliq.2022.119549
Development of a Fully Water-Dilutable Mint Concentrate Based on a Food-Approved Microemulsion
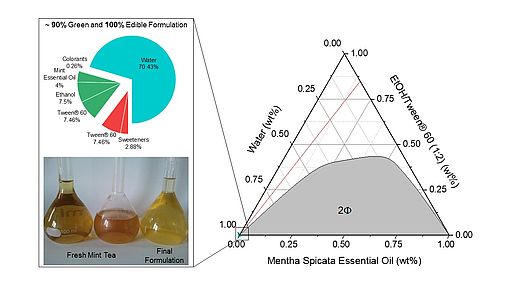
Mentha spicata L. disappears in winter. The lack of fresh mint during the cold season can be a limiting factor for the preparation of mint tea. A fresh taste source that can be kept during winter is mint essential oil. As the oil is not soluble in water, a food-approved, water-soluble essential oil microemulsion was studied, investigating different surfactants, in particular Tween® 60. The challenge was to dissolve an extremely hydrophobic essential oil in a homogeneous, stable, transparent, and spontaneously forming solution of exclusively edible additives without adulterating the original fresh taste of the mint. Making use of the microemulsions’ water and oil pseudo-phases, hydrophilic sweeteners and hydrophobic dyes could be incorporated to imitate mint leaf infusions aromatically and visually. The resulting formulation was a concentrate, consisting of ~90% green components, which could be diluted with water or tea to obtain a beverage with a pleasant minty taste. https://doi.org/10.1016/j.foodchem.2021.131230
Nanoscopic microheterogeneities or pseudo-phase separations in non-conventional liquids
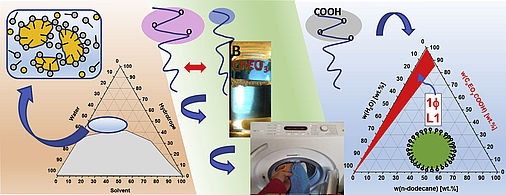
This article discusses new concepts in macroscopically monophasic colloidal systems, where entropy is a major driving force for very subtle interactions and structuring. First, we show how microemulsion-similar structures can be achieved with hydrotropes. These aggregates are less defined in structure and of shorter lifetime than classical micelles but still potentially useful microheterogeneities with internal interfaces. The other extreme case of strong interactions is given when cationic and anionic surfactants are mixed in equimolar ratios. It is well known that surfactants can be made more soluble when ethylene oxide groups are incorporated. This strategy is applied for such ‘catanionics’ to avoid surfactant precipitation. Finally, we consider the fact that ethylene oxide groups increase the size of the hydrophilic headgroups of carboxylates so that the geometrical constraints compel a direct spherical shape even in the absence of water. As a result, ‘water-free direct microemulsions’ with only charged surfactants and oil are conceivable.
https://doi.org/10.1016/j.cocis.2021.101535
Ionic Liquids Based on the Concept of Melting Point Lowering Due to Ethoxylation

Most of the commonly used Ionic Liquids (ILs) contain bulky organic cations with suitable anions. With our COMPLET (Concept of Melting Point Lowering due to Ethoxylation), we follow a different approach. We use simple, low-toxic, cheap, and commercially available anions of thex type Cx(EO)yCH2COO– to liquefy presumably any simple metal ion, independently of its charge. In the simplest case, the cation can be sodium or lithium, but synthesis of Ionic Liquids is also possible with cations of higher valences such as transition or rare earth metals. Anions with longer alkyl chains are surface active and form surface active ionic liquids (SAILs), which combine properties of ionic and nonionic surfactants at room temperature. They show significant structuring even in their pure state, i.e., in the absence of water or any other added solvent. This approach offers new application domains that go far beyond the common real or hypothetical use of classical Ionic Liquids. Possible applications include the separation of rare earth metals, the use as interesting media for metal catalysis, or the synthesis of completely new materials (for example, in analogy to metal organic frameworks). https://doi.org/10.3390/molecules26134034
Promising “green” solvent obtainable from woods & grasses
The molecule “γ-valerolactone“ (GVL)” can be readily synthesized from cellulosic biomass and could be used in a variety of commercial products, whilst having the potential to be even utilized in large-scale chemical processes. Additionally, it exhibits a very low toxicity towards aquatic environment and is readily biodegradable. These results were recently published in the leading journal for sustainable sciences “Green Chemistry” by the chair of Prof. Kunz in cooperation with researchers from TU Dresden. The article appeared on the cover of the respective issue and was featured for both the collection “2021 Hot Green Chemistry Articles” as well as the “Green Chemistry Editor’s Choice”. For the press release (in German), cf.: Link to press release.
In the article, the ecotoxicity of GVL towards aquatic plants, bacteria, invertebrates, and a vertebrate cell line was concluded to be very low. The lactone was also shown to be completely biodegradable within a month. Further, the solvent properties of GVL were modelled based on Hansen Solubility Parameters, COSMO-RS calculations and existing literature about GVL to evaluate potential applications of this “green solvent”. As a result, GVL could be shown to represent a promising substitute for several highly polar, aprotic solvents, such as the reprotoxic compounds N-methyl-2-pyrrolidone (NMP) and dimethylformamide (DMF). It was concluded to be of interest as a sustainable and less toxic solvent in the manufacture of certain polymers or pharmaceuticals, as a cleaning agent in various paint and coating formulations, as well as a solubilizer in cosmetics, pharmaceuticals, or agrochemicals.
Based on the promising results, the chair of Prof. Kunz is currently working together with an industrial company to build a pilot plant with a capacity of 2000 tons/year. In case of a higher demand, the production could then be boosted up to several thousand tons per year.
Link to the original article:
The green platform molecule gamma-valerolactone – ecotoxicity, biodegradability, solvent properties, and potential applications”, Green Chemistry (2021)