Current topics at the Department of Physical Chemistry II
Sustainable Chemistry – our Activities

Here is a selection of our research projects related to sustainable chemistry:
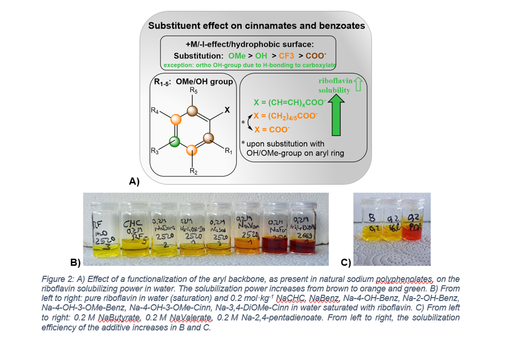
- Use water as a potentially “green” solvent for chemical products and reactions of industrial relevance: we discovered green additives and develop general concepts that allow the solubilisation of hydrophobic agents (reactants and catalysts) in water. We also apply this approach to improve water solubilities of vitamins, natural aroma molecules and natural antioxidants. Further, we design the structures of the aqueous solutions to boost chemical reactions in them, to prepare tailor-made solutions for best yields and optimum catalyst activity. To this purpose, we collaborate with colleagues in organic chemistry in Academia and Industry. Results are documented in several publications. Here is a very short selection:
- N. Ulmann et al., Self-association as a solubility limiting factor of riboflavin in aqueous media, Physical Chemistry Chemical Physics 26 (2024) 18930-18942; https://doi.org/10.1039/D4CP02074J.
- Adrien Fusina et al. Hydrotropic and solvent strategies to enhance the solubilization of polyphenols in relation to their chemical structure, Journal of Molecular Liquids 408 (2024) 125321; https://doi.org/10.1016/j.molliq.2024.125321.
- M. Schmidt et al., Aromas: Lovely to Smell and Nice Solvents for Polyphenols? Curcumin Solubilisation Power of Fragrances and Flavours Molecules 29 (2024). 294. https://doi.org/10.3390/molecules29020294
- N. Ulmann et al., Investigation of the salting-in/-out, hydrotropic and surface-active behavior of plant-based hormone and phenolic acid salts, The Journal of Colloid and Interface Science 641 (2023) 631-642. https://doi.org/10.1016/j.jcis.2023.03.077
- A. Fusina et al., Enhancement of water solubilization of quercetin by meglumine and application of the solubilization concept to a similar system, The Journal of Molecular Liquids 368 (2023) 120756. https://doi.org/10.1016/j.molliq.2022.120756
- M Giedyk et al., Photocatalytic activation of alkyl chlorides by assembly-promoted single electron transfer in microheterogenous solutions, Nature Catalysis 3 (January 2020), 40-47. doi:10.1038/s41929-019-0369-5.
- E. Hofmann et al., Sustainable cascade reaction combining transition metal-1 biocatalysis and hydrophobic substrates in surfactant-free 2 aqueous solutions, Chemical Engineering Journal 472 (2023) 144599. https://doi.org/10.1016/j.cej.2023.144599
- J. Blahnik, et al., Microemulsion and microsuspension polymerization of methyl methacrylate in surfactant-free microemulsions (SFME), The Journal of Colloid and Interface Science 648 (2023) 755-767. https://doi.org/10.1016/j.jcis.2023.06.025
- Y.-M. Tian et al., Enforced Electronic-Donor-Acceptor Complex Formation in Water for Photochemical Cross-Coupling, Angewandte Chemie Int. Ed. 62 (2023) e202218775. https://doi.org/10.1002/anie.202218775.
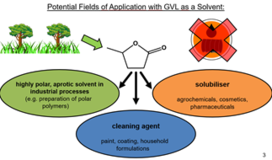
- Development and valorisation of new green, biogenic solvents as substitutes of toxic organic solvents. Among others, we could show that biogenic (and even drinkable!) gamma-valerolactone (GVL) (produced from hemicellulose, a waste product of cellulose valorisation) can replace popular but toxic solvents such as DMF or NMP, used in large quantities in industry. It can be even used to dissolve valuable polymers thus paving the way to their recyclability. We could even persuade an industrial company to produce thousands of tons of GVL at a very competitive price so that our discoveries will come to an industrial level soon.
In a very recent project, we try to replace chlorinated solvents which are still widely used, e.g., in chemical textile cleaning (“dry cleaning”), but even for caffein extraction, by environmentally more benign solvents.- K. Häckl and W. Kunz, Some aspects of green solvents, Comptes Rendus de l’Académie des Sciences (France) Chimie 21 (2018) 572-580.
- F. Kerkel et al., The Green Platform Molecule Gamma-Valerolactone – Ecotoxicity, Biodegradability, Solvent Properties, and Potential Applications, Green Chemistry 23 (2021) 2962-2976; https://doi.org/10.1039/D0GC04353B.

- We are very much involved in the use of plant constituents and residues and their selective, “green” extraction. We found that natural antioxidants can allow us to extract and solubilise other valuable plant ingredients. In several projects, partly still on an academic level, partly together with industrial partners, we develop very new concepts that we discovered, up to an industrial level. We focus especially on the valorisation of plant waste.
- A. Chemat et al., Coffee byproduct silverskin valorization, Molecules 29 (2024) 1318-1333. https://doi.org/10.3390/molecules29061318.
- C. Ernst et al., Valorisation of Hop Residues (Master Thesis 2024).
- P. Degot et al., Curcumin Extracts from Curcuma Longa – Improvement of Concentration, Purity, and Stability in Food-Approved and Water-Soluble Surfactant-Free Microemulsions, Food Chemistry 339 (2021) 128140. https://doi.org/10.1016/j.foodchem.2020.128140
- V. Huber et al., NADES-based surfactant-free microemulsions for solubilization and extraction of curcumin from Curcuma Longa, Food Chemistry 335 (2021) 129624. https://doi.org/10.1016/j.foodchem.2021.129624.
- V. Huber et al., Towards a Sustainable and Green Extraction of Curcuminoids Using the Essential Oil of Cinnamomum Cassia, Sustainable Food Technology (2023); DOI: 10.1039/d2fb00026a
- E. Hofmann et al., Novel green production of natural-like vanilla extract from curcuminoids, Food Chemistry 417 (2023) 135944. https://doi.org/10.1016/j.foodchem.2023.135944
- Adrien Fusina et al. Hydrotropic and solvent strategies to enhance the solubilization of polyphenols in relation to their chemical structure, Journal of Molecular Liquids 408 (2024) 125321; https://doi.org/10.1016/j.molliq.2024.125321.
- Michael Schmidt et al., Aromas: Lovely to Smell and Nice Solvents for Polyphenols? Curcumin Solubilisation Power of Fragrances and Flavours Molecules 29 (2024). 294. doi.org/10.3390/molecules29020294
Nadja Ulmann et al., Investigation of the salting-in/-out, hydrotropic and surface-active behavior of plant-based hormone and phenolic acid salts, The Journal of Colloid and Interface Science 641 (2023) 631-642. https://doi.org/10.1016/j.jcis.2023.03.077
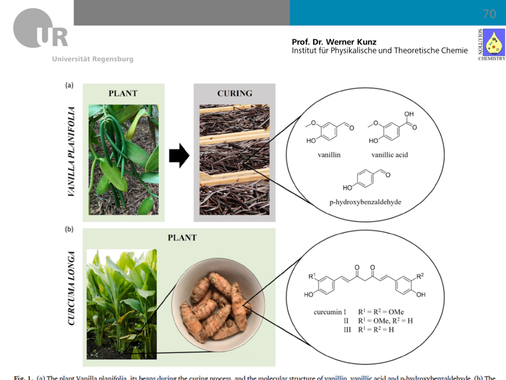
- Inorganic chemistry involving metals is often related to very high temperature and consequently is very energy consuming, especially on an industrial level. We invented simple, cheap, and environmentally friendly metal salts that are liquid at room temperature. Together with an industrial partner, we use them now to make industrially relevant processes such as coating or other metal processing at room temperature instead of heating to 600-1500°C.
- M. Rothe et al., Ionic Liquids based on the Concept of Melting Point Lowering due to Ethoxylation, Molecules 26 (2021), 4034. https://doi.org/10.3390/molecules26134034.
- M. Rothe et al., Liquids [M3+] [A–]3 with three-valent cations and their possible use to easily separate rare earth metals, Chemistry – A European Journal 27 (2021) 1-8. https://doi.org/10.1002/chem.202101925
- Concrete is responsible for more than 7% of the worldwide carbon dioxide production. Together with a big German chemical company, we try to develop substitutes, in which the cement is directly based on carbon carbonate and not made from carbon oxide after burning and release of CO2.
- J. Schuster, Development of cement substitutes based on non-burnt CaCO3, (ongoing PhD thesis).
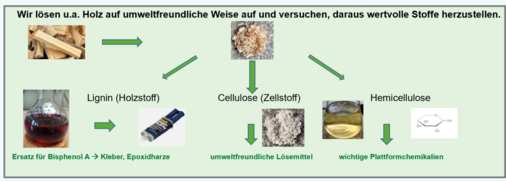
- Dissolution and Valorisation of Biopolymers. Nature provides a huge quantity of biomass that can be used as valuable materials. Over centuries, wood and cotton have been used, be it as construction material or to transform part of them to paper, textiles etc. We work on the separation of wood into cellulose, hemicellulose and lignin. We found a promising method to do that in a more environmentally benign way compared to current industrial processes. Further, we manage to cut down both hemicellulose and lignin to valuable monomers that can be used to make new polymer materials, e.g. bio-epoxy resins, bio-adhesives etc. This is done partly in collaboration with industry, partly within a Bavarian-Czech project. One of the goals is the valorisation of wood waste, another one to find an environmentally benign solubilisation process of cellulose and lignin. This concept could be even used to recycle cotton-based textiles. Further, we demonstrated that other important biopolymers such as chitin and suberin (a bio-polyester from tree barks) can be dissolved at low temperatures without destroying them and then be used to make new, interesting materials.
- A. Freyburger et al., Chitin coated cellulosic textiles as natural barrier materials, Lenzinger Berichte 94 (2018) 105-113.
- Y. Duan et al., Cellulose and chitin composite materials from a green solvent, Carbohydrate Polymers 192 (2018) 159-165.
- Y. Duan et al., Lignin/chitin films and their absorption characteristics for heavy metal ion Fe (III) and Cu (II), ACS Sustainable Chemistry and Engineering 6(5) (2018) 6965-6973.
- H. Garcia et al., Ex-situ reconstitution of the plant biopolyester suberin as a film, Biomacromolecules 15(5) (2014) 1806-1813.
- Moritz Schweiger, Lignin Isolation with GVL-Containing “Organosolv” Mixtures (Master Thesis 2023)
- Thomas Lang, The Quest of Bio-based Adhesive Monomers (Ongoing Master Thesis 2024)
- Development of new “green” and environmentally friendly surfactants and emulsifyers. We discovered that natural Stevia extract (the well-known sweetener) can be also used as a powerful emulsifier, especially in combination with natural soaps. We also investigate the properties of other natural molecules that may have a certain potential as surfactants. To this purpose, we closely collaborate with several industrial companies. Our aim is to replace synthetic and potentially unhealthy surfactants by natural, benign ones.
- S. Wolfrum et al., A Renaissance of Soaps? – How to make clear and stable solutions at neutral pH, Advances in Colloid and Interface Science 236 (2016) 28-42.
- S. Wolfrum et al., A New Strategy to Dissolve Long-Chain Surfactants in Water at Low Temperatures, Green Chemistry 24 (2022) 7675–7681. DOI: 10.1039/d2gc02460h.
- L. Braun et al., Alkylether derivatives of choline as cationic surfactants for the design of soluble catanionic systems at ambient conditions, The Journal of Molecular Liquids 370 (2023) 121033. https://doi.org/10.1016/j.molliq.2022.121033.
- P. Denk et al., The effect of ethanol on fibrillar hydrogels formed by glycyrrhizic acid monoammonium salt, The Journal of Colloid and Interface Science 630 (2023) 762–775. https://doi.org/10.1016/j.jcis.2022.10.138.

- Sustainable, “green” product formulations. In the framework of several industrial projects, we help our industrial partners to transform their products and processes into more environmentally benign ones. This is the case, in particular, for cleaning agents, cosmetics and perfumes, food and feed formulations, nutraceuticals, pharmaceuticals, paints and coatings, and in several other industrial sectors. In these collaborations, we do not simply propose optimised formula, but we try to develop tailor-made and at the same time generalisable concepts.
- C. Benkert et al., Development of a Fully Water-Dilutable Nanah Mint Concentrate Based on a Food-Approved Microemulsion, Food Chemistry 372 (2022) 131230. https://doi.org/10.1016/j.foodchem.2021.131230.
Solvents and hydrotropes
To solubilise a component in a solvent, the solvent must be well characterised. We participated in the proposition of a new type of solvent classification based on the COSMO-RS theory [168]. Together with our colleagues in Lille we also considered new types of solvents that exhibit surfactant properties. Such a hybrid class of liquids we called solvo-surfactants. In particular we were interested in glycerol derivatives [109] and so-called dowanols© [69,72,116,136,68,224] and related structures [134].
For a critical review of green solvents, see [261], also [241].
In this context it seemed of interest to illustrate the continuous cross-over of pure solvents to real surfactants and of mixtures between both [86,88,92,224,226]. Another interesting concept is the so-called facilitated hydrotropy [197, see also: M. Durand, A. Stoppa, V. Molinier, D. Touraud and J.-M. Aubry, J. Solution Chem., 2012, 41, 555–565]. Our present knowledge of hydrotropes is summarized in a review paper [237], see also [249][291][298][304].
The term “Hydrotrope” was coined by Carl Neuberg was coined more than a hundred years ago in a seminal paper. We recently translated it into English and critically commented it [298]. Hydrotropes are solubilisers of hydrophobic material in an aqueous solution. Recently, the term “biological hydrotrope” was used for molecules such as ATP that are able to inhibit the aggregation and fibrillation of protein aggregates leading to a solution of single protein molecules in an aqueous buffer. We critically discussed this point in terms of competing “Hofmeister” and “Neuberg” effects [291, 304].
We considered also the case of a charged hydrotrope and its influence on the structure formation in mixtures with water and oil [279].
The following picture is taken from paper [237].
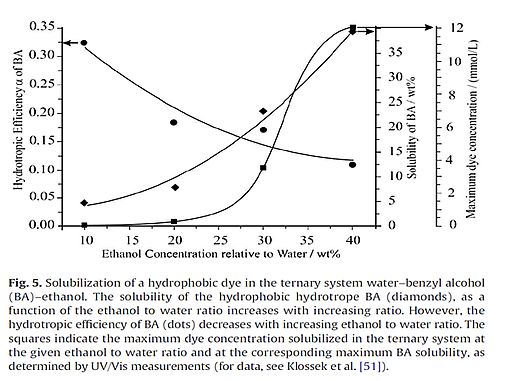
We also found that natural antioxidants such as the salts of ferulic acid, can act as potent hydrotropes, so playing a double role as stabilisers and solubilisers [325,338,341,342]. It should be noted that there are different mechanisms of hydrotropy. Not only they can act as small amphiphilic molecules to solubilise hydrophobic substances in water, but they can also act as anti-stacking agents that break the stacking of quite polar molecules in their crystalline form and as such enhance their water solubility. This is for example the case of indigo. Of course, there are also solvotropes, i.e., molecules that enhance the solubility of hydrophilic substances in a hydrophobic environment.
We also found that meglumine can be an interesting solubiliser both for antioxidants and to enable interesting organic synthesis [318,322,342]. Yet, the detailed mode of action of this hydrotrope is still a matter of debate.
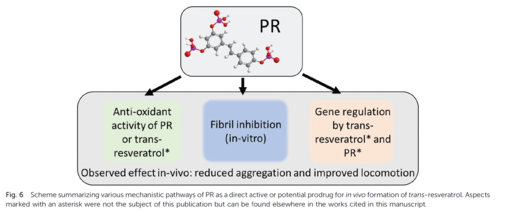
In the literature, so-called biological hydrotropes are discussed, as introduced by the group of Hyman [Patel, A., Malinovska, L., Saha, S., Wang, J., Alberti, S., Krishnan, Y., and Hyman, A.A. (2017). ATP as a biological hydrotrope. Science 356, 753–756.] Such molecules can render water-soluble various proteins, which tend to induce a phase separation of aqueous solutions. We investigated the possible mechanism and compared these biological hydrotropes to classical ones [291] and proposed further ones that even can help against Alzheimer’s disease [305]
(PR = phosphorylated resveratrol):
Concerning our efforts on the development of green solvents, we cite γ-valerolactone (GVL) as an example of a particularly promising solvent. GVL is a biobased solvent of very low toxicity (in fact even drinkable) that we characterised in detail over the last years [294], see also: https://onlinelibrary.wiley.com/doi/epdf/10.1002/nadc.20224119228 . We could convince the owners of an industrial company to build a factory to produce it on large scales for a very competitive price. The plant will be operational by the end of 2026.
Interestingly, binary liquid mixtures can be separated by soft centrifugation [293].
More information about our research activities on hydrotropes can be found in the chapter about Surfactant-free Microemulsions.
Surfactants, Micelles and Classical Microemulsions
So many research groups and industrial companies work on surfactants. Are there still any open questions? We thought that some aspects are still worth being studied:
- How to decrease the Krafft temperature of surfactants? Our idea was to use choline as a natural, biological cation that significantly reduces the solubilisation temperature of surfactants [130,144,194,207,223]. This is true for carboxylates and alkylsulfates, the latter being much less sensitive to water hardness [P5]. To further strengthen the effect, ethylene glycol (EO) groups can be inserted in the choline cation [315], see also the paragraph about specific ion effects.
- How to make olive oil and related fats miscible with water? To this purpose we studied so-called extended surfactants and indeed could make clear stable mini-emulsions with a minimum amount of surfactant [158,164,169,183].
- How to achieve the spontaneous formation of vesicles as simply as possible and with biocompatible surfactants? This question was considered in several papers [110,113,135]. In this context we also studied mixtures of cationic and anionic surfactants [128,143,145,148,154,311].
- We strongly believe that there is a future for soap-based surfactant systems [205] and that the inherent shortcomings (stable only at high pH, salt sensitivity, high Krafft points) can be overcome by appropriate formulations with other green ingredients. Currently, patents are filed to protect our efforts in this direction. A review paper on this subject can be found here [244]. In this context, we found that in particular the molecule rebaudioside A (the Stevia sweetener) is a very good co-surfactant and may be used as such in many formulations [244,230].
- Can we make new types of cationic surfactants with special properties [320]?
Micelles and microemulsions are also classical systems that have been studied thousands of times. We asked following questions:
- How to characterise microemulsions by classical scattering techniques [43,62,58,97] and less common techniques such as dielectric relaxation spectroscopy [46,47,57,79,85,98] under the guidance of our specialist Prof. Richard Buchner, and some other techniques [56,59]?
- What is the interplay between these structures and enzymatic reactions? How can such reactions be optimised by an appropriate design of nano-structured solutions [39,40,41,48,50,84,87,91,132]?
- Can enzymatic reactions also be performed in microemulsions in supercritical CO2 [51, 95]?
- How to make green microemulsions [212,184,198,197,199,204,203,307]?
- How to conceive microemulsions for well-defined purposes, for example for drug delivery [203] or for magnetic systems [189] or for food additives [230,307] or metal extraction [268]? In a recent collaboration with the group of Burkhard König from the Organic Chemistry Department, we could show that the finetuning of micellar systems can significantly improve photocatalytic reactions [278]:
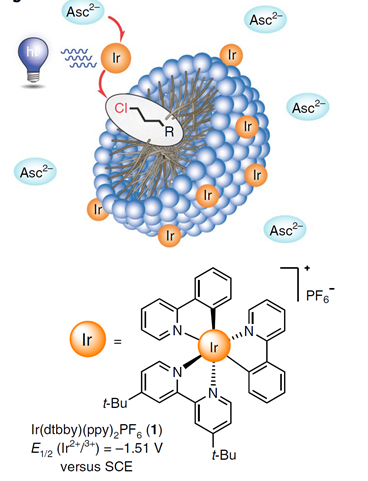
- Can we develop a theory to predict the influence of additives on the viscosity of surfactant+salt solutions? This was achieved by a collaboration with Wolfang Fieber from Firmenich, Geneva and Thomas Zemb, ICSM, France [269][292]. Note that the same approach can be used to predict clouding phenomena [270].
- Further work on micelles and microemulsions is published in [317,332,355].
- A dream came true: we produced, for the first time a so-called biliquid foam, meaning thermodynamically stable hydrophobic nanocapsules in an aqueous environment [349] as a stable alternative to nanoemulsions and made in a simple way with easily available and cheap surfactants.
In this context, we particularly acknowledge the long-standing cooperation with Prof. Thomas Zemb and the members of the institute of separation chemistry in Marcoule, France (ICSM) that he founded, in particular with Dr. Olivier Diat and Dr. Pierre Bauduin.
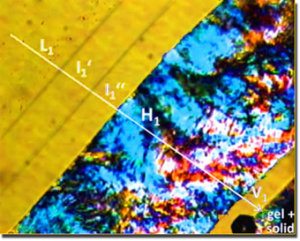
Penetration scan of ChC12 at 20°C acquired at 100x magnification between half-crossed polarizers, showing the following sequence of mesophases: L1, I1', I2'', H1, V1 and a gel+solid region. [173]
For some new ideas, see also [303] and [280].
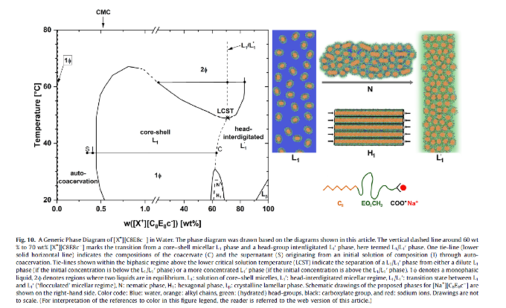
Recently, we reported on a new discotic lyotropic nematic phase made of bicelles formed by mixing octaoxyethylene octyl ether carboxylic acid (C8E8CH2COOH) and dioxyethylene oleyl ether carboxylicacid (C18:1E2CH2COOH) in water [332,336]. The following picture is taken from [332]:

For the astonishing phase behaviour of the single surfactant components (Akypos™), see [310]:
Surfactant-free Microemulsions(SFME) – The pre-Ouzo Effect
In the last years, we focused on a fascinating new subject, which is the microemulsion-like structuring of mixtures of two immiscible solvents with a third one, which is miscible with both of the two other ones. Such a structuring always occurs when the mixture is not too far away from the dephasing boundary. A typical example is the mixture of water, anethole (anise camphor) and ethanol, the well-known Ouzo liquor. If too much water is added, the mixture gets turbid and a very stable emulsion is formed. This strange finding of a surfactant-free, stable emulsion is called the Ouzo-effect. We call the pre-Ouzo effect the phenomenon that even in the homogeneous monophasic region of the phase diagram well-defined microemulsions are formed without surfactants. In analogy to classical microemulsions, direct, inverse and bicontinuous structures can be found. It should be stressed that such structures have been postulated and discussed in literature since at least 40 years, but widely neglected by solution and colloidal chemists. We have started detailed studies on these systems [191,179,205,206,216,219,225,228,229,233,240,242,284,285,295,297,303], because the understanding of this effect has significant consequences for product formulation, but it is also essential to understand the onset of structuring caused by very subtle, week interactions [293]. Our research on this topic is done in close collaboration with Prof. Thomas Zemb and Dr. Olivier Diat at ICSM, Marcoule, France and Prof. Dominik Horinek in our institute. Thomas Zemb and our Australian colleague Stjepan Marčelja from ANU, Canberra, developed with us a generalised DLVO theory to explain and quantitatively predict this pre-Ouzo structuring [236]. The following figure is taken from [242]. Remarkably, the nanoscopic structuring in SFMEs can be modelled and even partially predicted by a modified COSMO-RS approach [272].
We acknowledge the cooperation with Prof. Andreas Klamt, who is honorary professor in our institute and the inventor of the very useful modelling tool COSMO-RS, and we acknowledge also the fact that the company Dassault Systèmes–3DS provides us for free with the corresponding software.
BIC: bicontinuous
![]() : critical point
: critical point
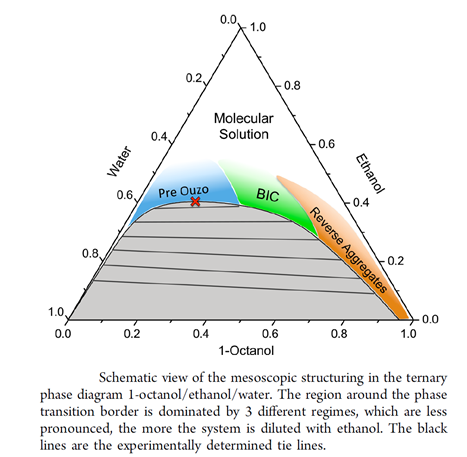
First attempts of possible applications of SFME are presented in [248,249,255,260,266,328,333,329], see also [303,331].
Plant Extraction
Having acquired a significant knowledge in solvent and solution chemistry and being interested in Green Chemistry, it is a natural step to apply our knowledge to the field of plant extraction processes. In the framework of several master and PhD theses, we considered plant extraction with classical methods (steam and Soxhlet extraction, maceration etc.), but also extraction with supercritical CO2 and “green” Ionic Liquids. For the moment, we focus on rhizomes of Iris germanica and Iris pallida, where we consider not only optimized green extraction processes, but also the optimization of the conversion of Iridals to Irones, the valuable and very expensive perfume molecules in the rhizomes. We have a fruitful collaboration with the French company Phytotagante in Toulouges, further with a big perfume producer and with the laboratory "GREEN" under the direction of Prof. Farid Chemat at the University of Avignon [252]. During his six-month stay at the University of Avignon, France, in the lab GREEN of Professor Farid Chemat, Prof. Kunz also participated in several other projects concerning plant extraction.
We also consider Rosemary extraction [238] and its optimisation and formulation for cosmetic products as well as other plants such as Roses (where hexane is replaced by environmentally friendlier solvent systems) and plants containing promising and low toxic saponis. We also developed a new strategy to extract Curcumin from Curcuma Longa and to dissolve significant amounts of it with high purity in aqueous and water dilutable solutions [284,285,288,289,297,301,307,313].
Further, we are interested in stabilising Latex out of Caucasian dandelion [257, 312].
Several new inventions are summarised in pending patents in the domain of plant extraction and valorisation through green processing.
More recently, we combined our efforts of extraction and formulation to optimise solubility, stability and extractability, essentially of curmuminoids out of Tumeric powder essentially using surfactant-free microemulsions, NADES and convenient hydrotropes [284,285,288,289,297,301,307,313,314,321,325], see also [340]. We also worked on the extraction of caffeine [338,344] and of extraction, solubilisation and application of various other natural substances, in particular antioxidants [318,325,337,342,350,352,357].
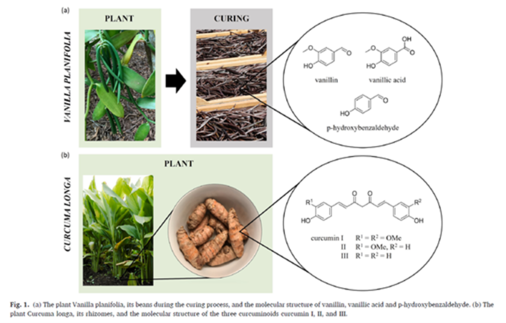
Natural vanillin is very expensive. We produced it in a natural process from curcuma [324]:
Solubilisation of Biomass
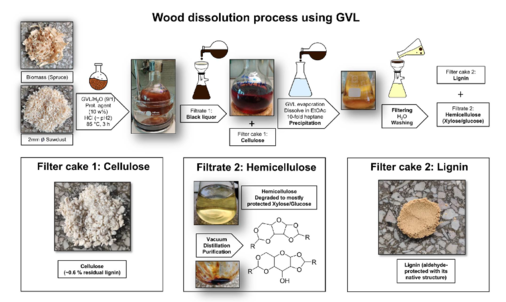
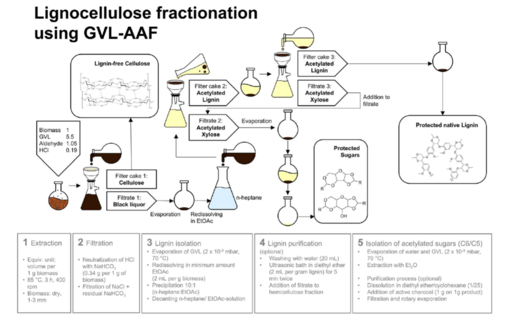
Cellulose, hemicellulose and lignin are the most important biopolymers in wood and most of the other plants. Together, they form a nearly infinitely available biomass that will be increasingly important for chemistry, unless we will continue to use petrol. It is essential to find simple and also biocompatible, sustainable solutions to separate and dissolve these biopolymers and also to cut down lignin to the inherent monomers that may serve as valuable starting material for modern and sustainable products. First attempts into this direction are published in [358,359]. Others will follow also as patents, which will show how the well-established but environmentally questionable viscose process can be replaced be modern sustainable approaches.
Picture taken from [359]:
Formulation Chemistry
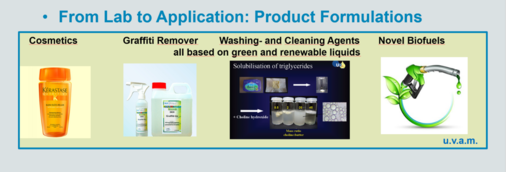
We take profit of our knowledge on complex solutions to conceive finished products ready to use. Examples are high-performance shampoos, environmentally friendly shaving foams, green anti-graffiti solutions, deodorants [151,152,174,219], green high performance oil dissolvers, but also food formulations. The latter is part of our effort to use plant extracts and to convert them to formulated products. Recently we succeeded in dissolving the very hydrophobic essential oil of the Arab Nanah mint tea in a drinkable microemulsion concentrate that can be diluted with water to get a clear stable tee of excellent taste.
For our anti-graffiti product, see http://www.skh-gmbh.de/graffiti-entfernen.html
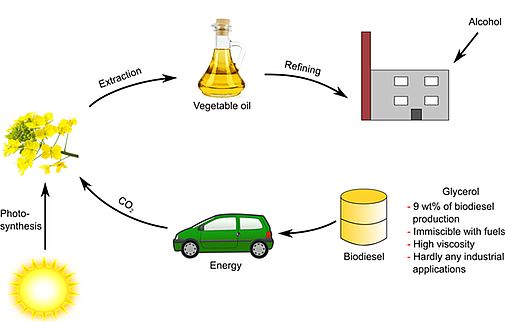
We also start to formulate new types of biofuels with a significant part of unmodified plant oils mixed with diesel and biodiesel and optimized with respect to the required conditions for fuels by using new types of green additives [234,240,250,265,276,P13]. In particular, we could show that we are able to stabilize vegetable oils with natural antioxidants at a very competitive price and at concentrations as low as the synthetic and toxic BHT and TBHQ [282]. A related patent is filed [P13].
Note that more recently, we worked with different companies on so-called bioactive glass. This material, when dispersed in water, exhibits high pH-values at its surface, a phenomenon that can be used to make new cosmetic formulations at neutral bulk pH values and only high values at the contact with surfaces. First formulations based on this principle are on the market.
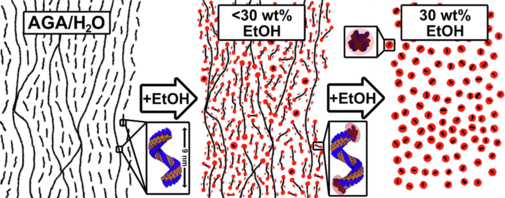
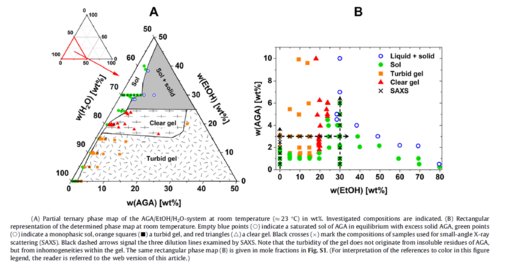
An interesting solubilisation, encapsulation and transport system is a hydrogel formed by glycyrrhizic acid monoammonium, in which both hydrophilic and hydrophobic agents can be solubilised and stabilised together. We investigated, how different alcohols influence its structure [319], see also next figures:
Aqueous and Non-queous Electrolyte solutions
As a continuation of Josef Barthel’s work (Josef Barthel is the founder of the chair of physical chemistry II at Regensburg university), we pursued the study of the structure and thermodynamics of non-aqueous, mostly electrolyte, solutions or mixtures of water with solvents [11,12,13,19,65,70,73,81,82,83,89,99,136,146], among others with Pierre Turq and his group in Paris. We also have a long-standing collaboration with our Ukrainian colleague Elena Tsurko from Charkiw University [117,126,147,190,220,227,235,246,262,271,273,330] and more recently with the group of Prof. Sadowski and Christoph Held at TU Dortmund [210,246,247].
The subject is the determination of thermodynamic properties of aqueous amino acid-salt solutions and specific-salting in and out behaviour of ingredients in aqueous systems, with a special regard to salting-out processes (extraction and purification) of biotechnologically produced products.
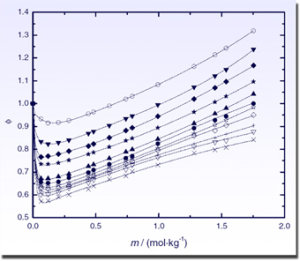
Experimental osmotic coefficients of (LiBr + DMAc) at different temperatures. The theoretical curves are generated using the extended Pitzer model of Archer. [82]
Specific Ion Effect, Hofmeister Series, Salting-in and Salting-outs
A long time ago, Prof. Kunz learnt to model aqueous and non-aqueous electrolyte solutions [10,12,16,17,22,29,30,32] both in Regensburg and under the guidance of Pierre Turq in Paris. Whereas first a relatively complex solvent-averaged modelling was intended, we turned later on to efficient working equations for engineers [49,53,67,100,104,123]. We acknowledge the tremendous work done by Jean-Pierre Simonin at the University Pierre et Marie in Paris.
Inspired by Prof. Barry Ninham (ANU Canberra, Australia) during his six-month stay in Regensburg in 2004, we began to get interested in specific ion effects. At first, we followed the idea of dispersion forces to describe these effects [61,74,75,77,80].
However, we soon realized that the problem is much more complex. Therefore, we went back to experiments and considered specific ion effects in a multitude of systems, e.g. in “simple” electrolyte solutions [90,94,103,108], surfactants systems (association colloids) [125,141,143,226,227], enzymatic systems [76,96] and on biological membranes [114]. The collaboration with Pavel Jungwirth and his group as well as with Roland Netz and his group (especially Dominik Horinek, and Joachim Dzubiella) and finally discussions with Kim D. Collins have been of great importance for us to understand these effects [101,105,106,118,124,127,129, 131,141,153,155,157]. In this context, we acknowledge our fruitful collaboration with Prof. Gordon Tiddy, Manchester university [195,215], where we could show the usefulness of NMR quadrupole splitting. Finally, we could even use our knowledge on specific ion effects to specifically design new surfactants [130,144].
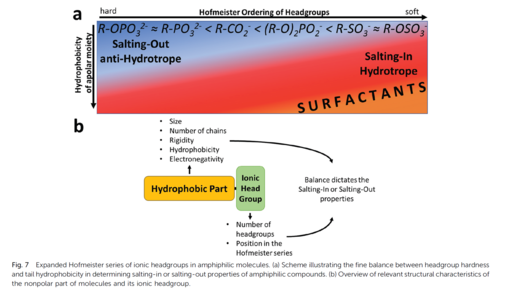
More recently, we came back to questions related to salting-in and -out effects of salts and also other ingredients that may fit into a generalized Hofmeister series [226,253,290]. Of particular interest is the continuous cross-over of ion effects to hydrotropic effects and hydrophobic/hydrophilic effects, when short, charged, amphiphilic molecules are considered [283].
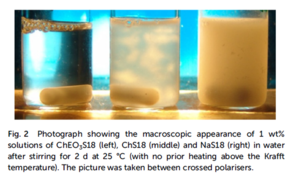
In the section about COMPLET Ionic Liquids, we report on the liquefaction of salts by introducing ethylene glycol (EO) groups into the anion so that nearly every metal cation, independently of its charge, can be part of a salt that is liquid at room temperature. The same strategy can be also applied to liquefy anionic surfactants by introducing EO groups into the cation, especially by linking them to choline cations [315]:
 |  |
Ionic Liquids and Deep Eutetics
Having worked for a long time with electrolytes, solvents and surfactants, it was natural to get interested in Ionic Liquids, i.e., salts that are liquid below 100°C or even at room temperature. In contrast to many other groups, we concentrated on two questions:
- How can we conceive Ionic Liquids that are not based on the classical imidazolium or other organic cations? In this context we had two major achievements: the discovery that short-chain alkyl-oligoether carboxylates are liquid at low temperature even with sodium as counterion and second that choline or carnitine derivatives [260] as counterion can also lead to Ionic liquids when combined with convenient middle- to long chain carboxylates [P3,142,163,171,259].
- Can we incorporate Ionic Liquids in micellar and microemulsion system [112] to make liquid systems over wide temperature ranges? This question we answered in details in the following papers:[139,156,160,161,162,163,166,172,175,176,182].
In a very fruitful collaboration with the Lisbon group of Dr. Cristina Pereira it was possible to characterize the solubilisation of suberin and the resulting reconstituted bio-polyester films out of a “drinkable” Ionic Liquid [208,211,217]. Suberin is the main polymer constituent of cork. This is the first bio-polyester material that is dissolved in and reconstituted from a green Ionic Liquid.
In collaboration with Prof. Rataj in Lille, France, and with the company A.R.D. as well as with Prof. Francois Jérôme, Université de Poitiers, we investigated in how far cellulose can be dissolved in environmentally friendly Ionic Liquid mixtures [213].
Note that even chitin can be solubilized in Ionic Liquids. We used this phenomenon to make hybrid materials of chitin with cellulose [263,267] and lignin [264]. Also new types of lubricants can be made of Ionic Liquids [281].
Ionic Liquids are typical examples of low-melting solvents. Low-melting systems can also be achieved via specific interactions of several components. Examples are so-called Deep Eutectic Solvents (DES). We developed a strategy to merge the characteristics of Ionic Liquids with DES [218]. We also showed that mixtures of a choline salt with urea and with sugars have melting points even below 0°C [214]. With DES, even surfactant-free and water-free microemulsions can be made [225]. Finally, we could show how the principle of DES can be advantageously used for green chemical syntheses [245]. For DES, see also [274]. Note that natural DES (NADES) can also be helpful in extraction processes [297]. For a general introduction, see our book chapter: Verena Huber, Katharina Häckl, Didier Touraud, and Werner Kunz, Natural deep eutectic solvents: From simple systems to complex colloidal mixtures, in: Advances in Botanical Research, Volume 97, London, Elsevier, 2021, ISSN 0065-2296. https://doi.org/10.1016/bs.abr.2020.09.001
Unfortunately, the hype with Ionic Liquids is still ongoing and the huge number of papers mask the really relevant ones [241].
A NEW TYPE OF IONIC LIQUIDS
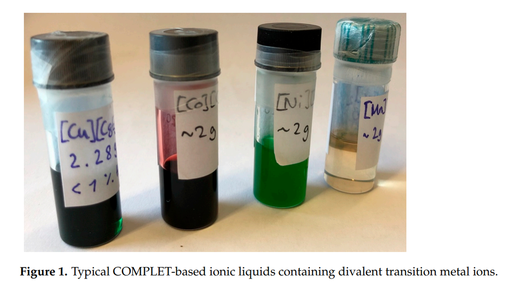
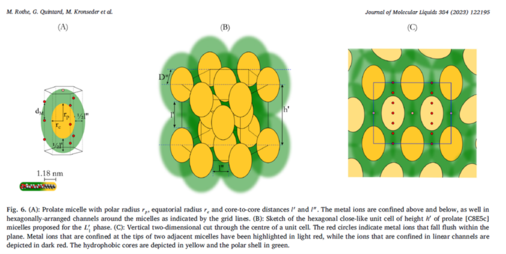
In the last years, we made a surprising discovery: our Concept of Melting Point Lowering (COMPLET), originally applied to simple singe-valent cations such as sodium (c.f. [Na][TOTO] [142,163,171]) can be extended to multivalent (two- and three-valent ones and maybe also to four-valent and higher-valent cations) so that we can obtain Ionic Liquids at room temperature of virtually any metal ion of the periodic table of elements [299] [300]:

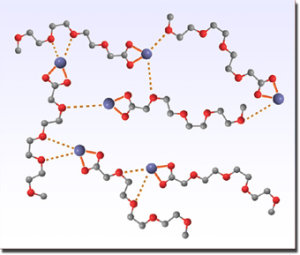
Sketch of the postulated crosslinked structure of liquid [Na][TOTO] with strongly bound Na+–––carbocxylate ion pairs and weaker Na+–––ether-oxygen interactions. [163]
These ILs are very simple to prepare, they are very stable (also against air and water), the used anions are of very low toxicity, and all are biodegradable, they are very cheap and as such of considerable importance for industrial applications. Currently, we make a systematic screening of such Ionic Liquids and investigate their practical relevance.
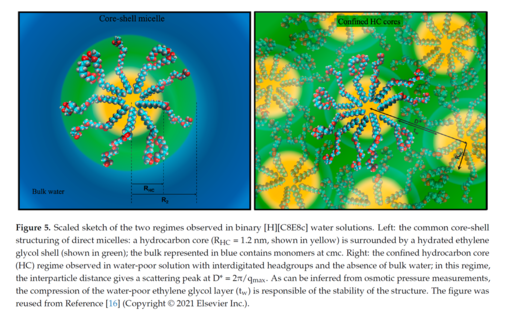
Note that there are also ionic liquid surfactants that are based on this concept [286,309]. They can even form direct micelles without or with only small amount of water [327]:
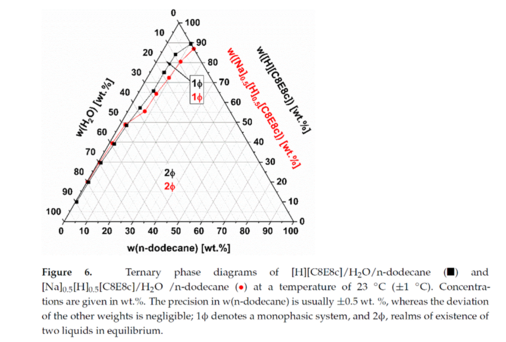
These direct micelles can be swollen, to a certain extent, by introducing an oil [299]:
Crystallisation, Nanocrystals, Biomorphs and Silica Gardens
Having heard fascinating lectures by Stephen Hyde, Canberra, and Juan-Manuel García-Ruiz, Granada, Prof. Kunz got interested in the world of complex crystalline structures made of simple ions in aqueous solutions such as silicates and carbonates. He wants to understand the origin of the phenomenon of biomorphs and the origin of curvature that simple ions create by an amazing self-aggregation process during crystallisation. Several papers came out of this research [115,119,121,133,137,140, 149,165,178,186,188,192,193,202,221,222,232]. A side-effect of this subject was the characterisation of chemical gardens, an old subject that even found entrance in school presentations [180,243,251,308,302,314]. In parallel, we could also make new core-shell-shell nanoparticles with silicate and carbonate [200]. Currently, we try to generalise this approach to the simple preparation of quantum dots.
A related research collaboration with the German Papiertechnische Stiftung (Foundation of Paper Industry) led to the fabrication of interesting hollow oxide microspheres [122,150].

Chemical gardens ... are beautiful structures that show fascinating membrane and diaphragm properties. [180]
More recently, we started also considering the precipitation of different calcium sulphate modifications [305].
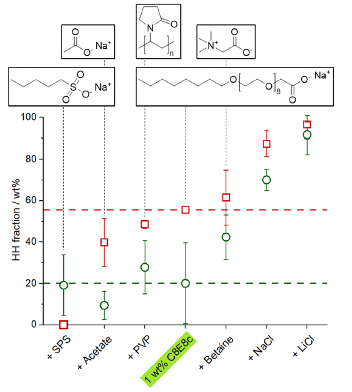
Results of calcium sulfate precipitation experiments performed in aqueous solutions of polyoxyethylene(8) octylether carboxylate (C8E8c) and different types of secondary additives (as indicated) at 25 (green circles) and 40 °C (red squares). Dashed horizontal lines represent the fractions of hemihydrate recovered at 1 wt% C8E8c without any second additive at the respective temperature. Dashed vertical lines serve as guides to the molecular structures of C8E8c and the used secondary organic additives. Detailed conditions for each precipitation experiment are given in Table S1 of the ESI. Note that the relatively large uncertainties for mixtures yielding low fractions of bassanite (i.e. < 40 wt%) arise mainly from isolation artefacts, which become significant under such "borderline" conditions, where small variations in temperature can readily cause (partial) phase transformation from bassanite to gypsum during workup. Symbols represent average values with their corresponding standard deviation obtained from at least two independent determinations.
See also [356]:

Further work on spontaneous stabilised nanoparticle formation can be found in [323].