
Current topics
Ion specific effects
Aqueous salt solutions
The most simple ion specific effect manifest in the surface tension of aqueous electrolyte solutions. In general, ions increase the surface tension in a specific manner. The traditional picture of the interface of electrolyte solutions is based on a thermodynamic analysis of the equilibrium surface tension isotherm. The increase in the equilibrium surface tension is then interpreted as an interfacial zone depleted by ions. Recently this picture has been challenged by molecular dynamics simulations which predicted that soft ions such as halides are enriched at the interface with a non-monotonic ion profiles. The key to an understanding of this apparent contradiction lies in a reconsideration of the meaning of thermodynamics. So it can accommodate several interfacial disrtibutions providing that the integral excess or depletion is in accordance to Gibbs equation. Therefore, direct experimental observations of molecular structure and energetic of ions in the interfacial region are required.
 |  |
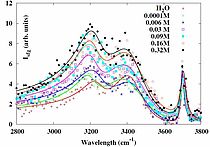 |
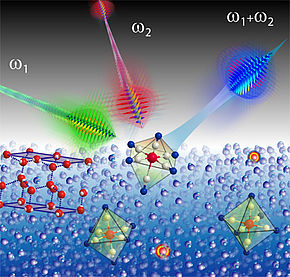
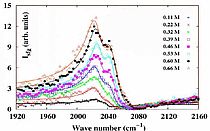
We used Infrared-Visible Sum Frequency Spectroscopy (IR-VIS SFG) to study the interfacial composition and structure of aqueous potassium thiocyanate electrolyte solutions. The IR-VIS SFG spectra reveal the propensity of the thiocyanate ions at the air-electrolyte interface.
They also give access to the vibrational features of the interfacial water which are affected by the presence of the ions. Polarization dependent measurements have been used for a determination of the orientation of the pseudo-halide anion. The combined data gives a picture of the interfacial architecture on a molecular scale. We believe our current study contributes towards better understanding of this biologically relevant chaotropic ion and water interactions at the interface. Further our work shows that the orientation of the anion is relevant and needs to be taken into account to get a full picture on the interfacial architecture.
To summarize, we have studied the aqueous solution containing thiocyanate anion using IR-VIS SFG spectroscopy. The dependency of the anion and the water features were monitored in dependence of the bulk concentration.
Ion profiles at a charged surface interfaces
Ion specific effects can be ordered in the so-called Hofmeister series which are a manifestation of the complex interplay of electrostatics, dispersion forces, thermal motion, fluctuation, hydration and ion size effects and the interfacial water structure Consequently the list of decorations and modifications of the original Poisson-Boltzmann equation is long in order to provide a more realistic picture.
The decisive information is completely contained in the ion distribution at an interface. We developed a simple experimental protocol based on ellipsometry and Second Harmonic Generation that provides insides in the prevailing ion distribution. A scientific highlight is the experimental evidence for an ion condensation at an interface just before the critical micelle concentration. The ion condensation can also be verified in the IR-VIS SFG bands of the interfacial water. The water dipoles adopt a preferential orientation in the electric field. SFG detects only the oriented water dipoles, hence, the SFG intensity can be related to the number of water dipoles contributing to the spectra. The interfacial water is a probe for the local electric field. This potential will be further utilized and explored.
A review on this topic: PDF download
Hubert Motschmann and Patrick Koelsch, Linear and Non-linear Optical Techniques to Probe Ion Profiles at the Air–Water Interface, in SPECIFIC ION EFFECTS edited by Werner Kunz, World Scientific Books, 2009.
Surface rheology
Surface rheology governs a great variety of interfacial phenomena such as foams or emulsions and plays a dominant role in several technological processes such as high speed coating. Its major difference with bulk rheology resides the high compressibility of the surface phase, which is the direct consequence of the molecular exchange between adsorbed and dissolved species.
 |
E(ω) = A (Δγ/ΔA)
|
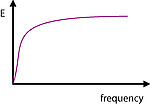
In analogy to bulk rheology, a complex surface dilational modulus, E, that captures surface tension changes upon defined area changes of the surface layer, can be defined. The module E is complex and the molecular interpretation of the dissipative process that gives rise to the imaginary part of the module is subject to some controversy.
The surface elastic modulus E is defined as the change in the surface pressure upon a relative area change of the surface area. It is a measure for the ability of the system to adjust its surface tension in an instant of stress. The experimental determination of the dilational modulus requires the measurement of the dynamic surface tension upon harmonic compression and expansion cycles of the surface layer.
E(ω,c) = ΙEΙ exp(iφ(ω, c))
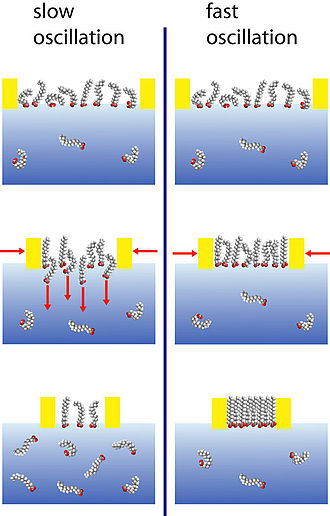
The surface dilational modulus is in general complex and depends on the frequency. The compression and expansion cycles of the surface layer disturb the prevailing equilibrium. As a consequence, amphiphiles must dissolve to the bulk or adsorb at the interface to restore the equilibrium coverage. It is important to compare the time frame of the internal relaxation processes with the one of the external perturbations. There is no resistance against area changes and the surface remains in equilibrium if the external frequency is on this scale small. On the other hand, at very high frequencies, the molecular exchange is suppressed and the adsorption layer behaves similar to an insoluble monolayer. The most interesting range is the intermediate mid-frequency range which is for soluble surfactants, around 1-500 Hz.
Several arrangements have been used for the measurement of the complex E-modulus, the classic experiment uses oscillating moving barrier devices. For technical reasons. the upper frequency limit is on the order of 0.01 Hz. A further common alternative is oscillating drop/bubble shape tensiometry. The shape of a drop is given by the balance between gravity and surface tension. The evaluation of the drop shape according to the Gauss-Laplace equation yields the surface tension and the corresponding surface area. This measurement scheme is implemented in many commercial tensiometer, however, a careful assessment of the limitations reveals an upper physical limit of about 1 Hz as discussed by Leser et al.
An interesting and superior alternative to oscillating drop tensiometry is capillary pressure tensiometry. A bubble is formed at the tip of a small capillary (diameter about 0.4 mm) and forced by a piezo translator in a well-defined oscillation. Instead of analyzing the drop shape, the pressure difference across the bubble interface is measured. This arrangement allows the precise measurement of the modulus E in the important mid-frequency range of 1-500 Hz with room for improvement in both directions. The complex surface dilational modulus can be obtained by analyzing the amplitude of the pressure response and the relative phase shift between the applied sinusoidal piezo voltage and the sinusoidal pressure response.
Surface rheology and foam stability
The imaginary part of the modulus can be interpreted as an intrinsic surface dilatational viscosity. The underlying dissipative process is the molecular exchange between adsorbed and dissolved surfactants. Some surfactant systems are purely elastic while others exhibit a crossover to a surface visco-elastic behavior. Our data indicate that the existence of an intrinsic surface dilatational viscosity is a prerequisite for the ability of a surfactant system to form a stable foam lamella. Hence, we are able to link foam stability to a fundamental system parameter. The surface viscosity damps mechanical distortions of the foam lamella and thus prevents film rupture.

