VITA
Short biography
Rainer Müller was born on December 22, 1968 in Schrobenhausen, Bavaria (Germany). He studied chemistry at the University of Regensburg where he graduated in February 1995. His diploma-thesis was entitled "Synthesis and physicochemical characterization of biologically degradable cationic polyelectrolytes" and was prepared at the Institute of Physical and Macromolecular Chemistry under supervision of Professor Klaus Heckmann. He obtained his PhD in February 1998 at the Laboratory of Interface Chemistry which was the follow-up institution after Professor Heckmann´s retirement. The PhD-thesis was entitled "Synthesis of corrosion-inhibiting coupling agents for microelectronic devices".
After attaining his doctoral degree, Rainer Müller was scientific co-worker at the Laboratory of Interface Chemistry where he established a research group focused on colloid and interface chemistry with special emphasis on biomaterials and tissue engineering. In December 2000 he became scientific assistant at the Institute of Physical and Theoretical Chemistry of the University of Regensburg headed by Professor Werner Kunz starting his habilitation work. He initiated several co-operations with the Departments of Trauma Surgery, Operative Dentistry, and Neurology of the University Hospital of Regensburg and other university institutes which were funded by the Deutsche Forschungsgemeinschaft (DFG) or by the Bavarian State Ministry of Sciences, Research and the Arts. In November 2009 he finished his habilitation-thesis entitled "Chemical modification of biomaterial surfaces and scaffolds to direct biological responses in regenerative medicine".
Since August 2011 Rainer Müller is senior research assistant at the Institute of Physical and Theoretical Chemistry of the University of Regensburg. He gives lecture courses, tutorials and laboratory classes for undergraduate students of chemistry, biochemistry and biology. In October 2016 he was appointed 'Außerplanmäßiger Professor' at the University of Regensburg.
Teaching
Teaching
Apl. Prof. Dr. Rainer Müller
University of Regensburg
Chemistry Undergraduates
- Tutorial Mathematical Methods in Physical Chemistry (2. Sem., summer term)
- Tutorial Thermodynamics Part 1 (2. Sem., summer term)
- Tutorial Chemical Kinetics (2. Sem., summer term)
- Tutorial Thermodynamics Part 2 (3. Sem., winter term)
- Tutorial Electrochemistry (3. Sem., winter term)
- Laboratory Course Physical Chemistry Part 1 (3. Sem., winter term)
- Seminar to Laboratory Course Physical Chemistry Part 1 (3. Sem., winter term)
- Laboratory Course Physical Chemistry Part 2 (4. Sem., summer term)
Chemistry Graduates
- Lecture Physical Chemistry of Biological Interfaces and Biomaterials (7. Sem., winter term)
- Laboratory Course Physical Chemistry Part 3 (Kurspraktikum, Vertiefungspraktikum), Adsorption Isotherms (8. Sem., summer term)
- Laboratory Course Formulation (8. Sem., summer term)
- Research Internship (7./8.Sem. winter or summer term)
Teachers of Chemistry
- Lecture and Tutorial Physical Chemistry – Introduction to thermodynamics, kinetics, surface chemistry and electrochemistry (3./5. Sem., winter term)
- Laboratory Course Physical Chemistry (4./6. Sem., summer term)
- Seminar to Laboratory Course Physical Chemistry (4./6. Sem., summer term)
Biochemistry and Biology Undergraduates
- Lecture and Tutorial Physical Chemistry – Introduction to thermodynamics, kinetics, surface chemistry and electrochemistry (3. Sem., winter term)
- Laboratory Course Physical Chemistry (4. Sem., summer term)
- Seminar to Laboratory Course Physical Chemistry (4. Sem., summer term)
Teaching
Privat-Dozent Dr. Rainer Müller
University of Regensburg
| Veranstaltungsnummer, Modul | Veranstaltung | Semester, Wochenstunden |
| 53281 – CHE-MSc-M 12 | Vorlesung Physikalische Chemie biologischer Grenzflächen und Biomaterialien | 7. Sem., WS, 3 SWS |
| 53360 – CHE-LA-M 10 | Vorlesung und Übungen Physikalische Chemie für Studierende der Biochemie, der Biologie und des Lehramts Chemie | 3./5. Sem., WS, 3 SWS |
| 53021 – CHE-BSc-M 06 | Übungen zu Thermodynamik 1 | 2. Sem., SS, 1 SWS |
| 53021 – CHE-BSc-M 06 | Übungen zu Thermodynamik 2 | 3. Sem., WS, 1 SWS |
| 53030 – CHE-BSc-M 07 | Praktikum Physikalische Chemie 1 | 3. Sem., WS, 4 SWS |
| 53031 – CHE-BSc-M 07 | Seminar zum Praktikum PC 1 | 3. Sem., WS, 1 SWS |
| 53550 – Biochemie-M 05 | Praktikum Physikalische Chemie für Biochemiker und Biologen | 4. Sem., SS, 3 SWS |
| 53551 – Biochemie-M 05 | Seminar zum Praktikum PC für Biochemiker und Biologen | 4. Sem., SS, 1 SWS |
| 53315 – CHE-LA-M 16 | Praktikum Physikalische Chemie für Studierende des Lehramts | 4./6. Sem., SS, 4 SWS |
| 53316 – CHE-LA-M 16 | Seminar zum Praktikum PC für Studierende des Lehramts | 4./6. Sem., SS, 1 SWS |
| 53130 – CHE-MSc-M 03 | Praktikum Physikalische Chemie 3 Versuch Adsorptionsisothermen | 8. Sem., SS, 10 SWS |
| 53270 – COSOM-M 04 | Praktikum Formulierung Versuche Rheologie, Schaumbildung, Phaseninversionstemperatur | 8. Sem., SS, 5 SWS |
| 53187 – CHE-MSc-M 12 | Forschungspraktikum Physikalische Chemie | 7./8. Sem., 10 SWS |
Research
Research in the Work Group of Rainer Müller, Department of Physical Chemistry
Contributions of Colloid and Interface Chemistry to Biomaterials Science and Regenerative Medicine
Biomaterial science aims to develop man-made materials and living tissues for replacing the physical and biological function of injured or diseased tissues in the human body. A broad understanding of material science and human biology is a prerequisite for the development of materials which not only exert certain structural and mechanical properties, but are also compatible with the biological system in which they are implanted. In addition, an understanding of the surface chemistry of materials is also very important since this is a main determinant for biocompatibility. The goal of material-based regenerative medicine has changed from using materials, which only match the physical properties of the replaced tissue combined with a minimal toxic response in the host, to the design of bioactive and biodegradable materials. Members of the so-called ‘third-generation biomaterials’ should instead be able to elicit specific desired responses from the surrounding tissues at the molecular level. Methods of colloid and surface chemistry play an important role in the design of new biomaterials, especially by improving the surface performance of materials and creating biocompatible three-dimensional guiding structures. Advances in surface science and molecular biology must be combined in order to understand how surface properties control the biological response of a tissue interacting with this surface.
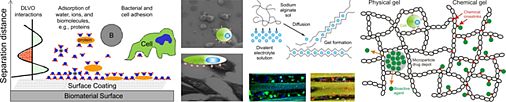
Fig. 1: Potential applications of colloid and surface chemistry in context with biomaterials science.
Influence of Biomaterial Surface Characteristics on Biological Processes
We investigate the influence of physicochemical surface parameters of biomaterials on the adsorption of proteins and the adhesion of eukaryotic and bacterial cells. Together with the Department of Operative Dentistry and Periodontology (UR) we developed new chemiluminescence- and fluorescence-based assays for the quantification of proteins and bacteria attached to surfaces of low specific area. In our group, silicon-based model surfaces with varying degrees of surface wettability, surface charge, and topography were prepared to study the impact of these parameters on biological systems interacting with these surfaces.

Fig. 2: Physicochemical properties of silicon-based model surfaces.
Using our model system, we identified certain surface chemical groups to which eukaryotic cell adhesion was different compared to bacterial cell binding. Surface modification with short-chain poly(ethylene glycol) moieties allowed adhesion of anchorage-dependent osteoblasts and fibroblasts, while binding of pathogenic bacteria was significantly inhibited. In contrast, surfaces modified with fluorocarbon moieties promoted attachment of bacteria but inhibited adhesion of eukaryotic cells. Moreover, we manufactured surfaces which clearly exerted antibacterial properties. Surface modification with cationic pyridinium group-containing molecules did in fact allow binding of oral bacterial strains but killed these bacteria almost completely on contact. We could show that even more than surface chemistry and topography, the adsorption of proteins to a substrate surface most significantly influenced the response of the contacting biological system. Pre-adsorption of cell adhesive proteins most often increased eukaryotic cell adhesion and subsequent cellular responses, although it also enhanced the potential of undesirable bacterial processes. In this context, surfaces pre-conditioned with saliva proteins were shown to increase the attachment of oral bacteria and, with the most definitive consequences, abolish the bactericidal efficiency of cationic surface modifications.

Fig. 3: Cell adhesion and bacterial viability on differently modified silicon-based model surfaces.
Based on these findings, follow-up research in our group focused both on the development of antibacterial surface coatings that additionally resist protein adsorption and on the surface modification of implant materials to specifically increase eukaryotic cell adhesion. These new surface coatings, which are based on dendrimers, are currently being investigated in cooperation with the Department of Oral Biology, State University of New York at Buffalo and the Division Cell Biology of the Center for Medical Research of the University of Rostock.
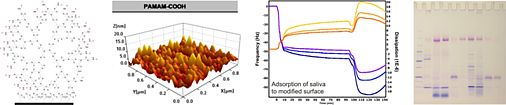
Fig. 4: Dendrimers as new type of biomaterial coating.
Literature: S. Stählke et al. Materials Science & Engineering C, 2019, 101, 190-203, H. Schweikl et al. Dental Materials 2013, 29, 1080-1089, V. Katzur et al. Journal of Colloid and Interface Science 2012, 366, 179-190, M. Eichler et al. Biomaterials 2011, 32, 9168-9179, R. Müller et al. Biomaterials 2009, 30, 4921-4929, R. Müller et al. Journal of Biomedical Materials Research A 2008, 84, 817-827, H. Schweikl et al. Journal of Material Science Materials in Medicine 2007, 18, 1895-1905, R. Müller et al. Applied Environmental Microbiology 2007, 73, 2653-2660, R. Müller et al. Analytical Biochemistry 2006, 359, 194-202.
Capillary Hydrogels for Neural Tissue Regeneration
We employ delicate colloid chemical procedures to prepare three-dimensional scaffolds exhibiting a highly exclusive porous structure. Although the natural polyelectrolyte alginate has been used in many biomedical disciplines for some time, the very specific feature this colloid system is able to exert has remained unrecognized in the field. It was the German colloid chemist Heinrich Thiele who discovered the formation of highly anisotropic capillary hydrogels, in which water-filled capillaries of round shape are aligned parallel to each other with a capillary density of up to 1000 per mm2, by a simple ion-driven process. We are the first to utilize this specialized structure for biomedical applications with the intention of preparing a guiding structure to conduct the regeneration of injured nerves. We could show that the variation of experimental parameters allowed us to specifically modify the anisotropic alginate gels with respect to their capillary diameter and capillary density. The capillary diameter can be defined within 10 and 200 µm and we contend that this parameter will exert a significant influence on the capability of capillary hydrogel-guided axon regeneration. In collaboration with the Spinal Cord Injury Center of the Heidelberg University Hospital we are currently investigating these gels with regard to their capacity to guide disrupted nerve axons in the central or peripheral nerve system.
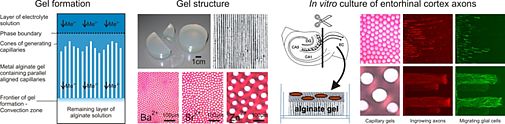
Fig. 7: Anisotropic capillary hydrogels as guiding structures for directed axon regeneration.
In addition to the unique physical properties of the highly ordered capillary system, further important properties concerning biomechanics, biocompatibility and bioactivity are also currently being investigated. Here again, we apply chemical crosslinking procedures to slow down biochemical degradation of the carbohydrate scaffold. Furthermore, we incorporate proteins of the extracellular matrix or other bioactive agents to enhance cell adhesion and provide a growth-supportive environment. All of these modifications are necessary for reaching our goal of establishing a new therapeutic strategy for the regeneration of nerves after peripheral nerve or spinal cord injury.
Literature: T. Schackel et al. Tissue Engineering A, 2019, 25, 522-537, S. Liu et al. Acta Biomaterialia, 2017, 60, 167-180, M. I. Günther et al. Acta Biomaterialia 2015, 27, 140-150, K. Pawar et al. Acta Biomaterialia 2015, 27, 131-139, K. Pawar et al. Acta Biomaterialia 2011, 7, 2826-2834, R. Müller et al. in Handbook of hydrogels: Properties, preparation and applications. Nova Science Publishers, Hauppauge, NY, 397-426, 2009, P. Prang et al. Biomaterials 2006, 27, 3560-3569.
Bioactivation of Metallic Implant Materials by Covalent Collagen Immobilization
In cooperation with the Department of Trauma Surgery (UR), the Department of Functional Materials in Medicine and Dentistry at the University of Würzburg, and the Department of Material Science at the Technical University of Dresden, surface modifications of metallic implant materials have been developed to improve the bioactivity of these materials. As determined from the previous project, for biomedical applications in which strong cell attachment and tissue integration is advantageous, such as for prostheses in contact with bone, the strategy of precoating these materials with layers of cell adhesion molecules seemed to be quite promising. The rationale behind the project was to test whether covalent immobilization of such adhesion-promoting molecules could be proven better than physical surface-adsorption for reducing the exchange and biodegradation of these proteins under physiological conditions. To this end, we developed and optimized strategies for the covalent binding of the extracellular matrix protein collagen to clinically relevant metallic materials. We examined stainless steel, titanium- and cobalt-based alloys which were first passivated by our research partners using surface deposition and electrochemical techniques and then subsequently bioactivated by covalent collagen coating.

Fig. 5: Covalent collagen coating to biomedical metals.
We found that immobilization of collagen was most effective using silane-coupling agents in combination with a mild crosslinking reagent which additionally stabilized the immobilized protein layer. Enzymatic digestion clearly displayed a higher stability of the covalently bound collagen layers compared to physically adsorbed collagen layers. Furthermore, we found that a high density of surface-bound coupling agents increased the stability of the covalently linked collagen. After coating metallic biomaterials with crosslinked collagen, an improved cellular response in the form of elevated adhesion and proliferation of human osteoblast-like cells and human mesenchymal stem cells was observed. While adhesion of eukaryotic cells was clearly increased, collagen modification of titanium surfaces reduced attachment of a typical pathogenic bacterium. In conclusion, the collagen coating developed in our group was able to positively affect two beneficial biological events which confirmed the successful bioactivation of metallic implant materials.
Literature: R. Müller et al. in Metallic biomaterial interfaces. Wiley-VCH, Weinheim, 88-92, 2008, R. Müller et al. BIOmaterialien 2007, 8, 40-45, R. Müller et al. Biomaterials 2006, 27, 4059-4068, R. Müller et al. Biomaterials 2005, 26, 6962-6972.
Biomimetic Scaffolds for Cartilage Tissue Repair
In collaboration with the Department of Trauma Surgery (UR) and the Department of Pharmaceutical Technology (UR), we examined the utility of scaffolds made from the natural constituents of cartilage tissue, i.e. hyaluronic acid and collagen, for articular cartilage engineering. We utilized a number of physicochemical methods to investigate the effects of material source, composition and chemical stabilization on the chemical and physical properties of these materials in an attempt to correlate them with their biological features. In our group, highest priority was given to the investigation of crosslinking procedures which stabilized the natural polymeric materials but still retained their intrinsic bioactivity.
We could identify and optimize one crosslinking procedure in particular which provided very good results with respect to biomechanical stability and enzymatic degradability as well as cell compatibility in terms of improved adhesion, proliferation, and differentiation of chondrocytes and mesenchymal stem cells. Moreover, this crosslinking system was even able to bond together native blocks of articular cartilage without reducing cell vitality, which may prove to be a promising tool for the application of in vitro-engineered cartilage tissue. The incorporation of a collagen constituent (gelatin) into a hyaluronan-based three-dimensional scaffold clearly initiated bioactive features with regards to the generation of a highly cartilage-inducing milieu characterized by promoting adhesion and differentiation of mesenchymal stem cells. The application of selected crosslinking procedures additionally enhanced the utility of the composite matrix as a biomimetic material for cartilage tissue engineering.
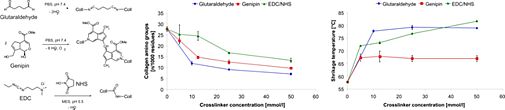
Fig. 6: Crosslinking of collagen monitored by free amino group content and thermal stability.
Literature: P. Angele et al. Journal of Biomedical Materials Research A 2009, 91, 416-427, C. Englert et al. Arthritis Research & Therapy 2007, 9, R47, P. Angele et al. Biomaterials 2004, 25, 2831-2841, P. Angele et al. BIOmaterialien 2003, 4, 11-18.
Monomolecular Coatings for Technical Applications
Self-assembled monolayers of long-chain organic molecules have been developed to act as corrosion inhibiting or adhesive surface coatings. In ceramic technology fine and finest powders with particle sizes far below 1 µm are required in an increasing manner because the properties of the ceramics depend on the microstructure which is influenced by the particle size of the starting powders. Due to their high surface to volume ratio these particles possess a large excess of surface free energy. While nanoscaled oxidic powders can be handled in the presence of air and water without major problems, the properties of nanoscaled non-oxidic powders like nitrides, carbides or carbonitrides change drastically in the presence of air and water due to oxygen uptake initiated by oxidation and hydrolysis. Furthermore, spontaneous inflammation of nanoscaled non-oxidic powders can be observed. In our project we synthesized long-chain aliphatic derivatives of primary amines, ethylenediamines, guanidines, nitriles, isocyanates and succinimides and applied them as coatings for TiN, TiC and SiCN nanopowders.

Fig. 8: Self-assembled monolayers as corrosion inhibiting coating for non-oxidic nanoparticles monitored by adsorption isotherms and thermogravimetric analysis in air atmosphere.
To increase the adhesion strength in polymer-packed microelectronic devices we synthesized new coupling agents which should be deposited as self-assembling monolayers between a copper lead frame and an epoxy molding compound. The coupling agents were preliminary chemisorbed at the surface of copper plates via special binding groups like thiol, disulfide, ethylenediamine and phthalocyanine. Binding to the epoxy resin was performed via a hydroxyl group. Linear hydrocarbon spacers with various chain lengths connected the copper- and epoxy-binding groups. X-ray photoelectron spectroscopy of the self assembled layers of the organic coupling agents confirmed its orientation at the metal surface. Thermogravimetric analysis was used to study the coating on corrosion inhibition. Shear tests clearly indicated that the coupling agents increase adhesion strength and are stable even in extreme humid and thermal conditions. Thus delamination of the microelectronic packages (‘popcorn effect’) was prevented.
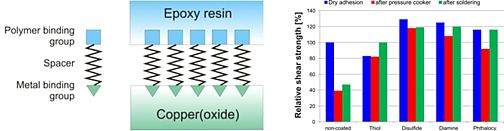
Fig. 9: Self-assembled monolayers as coupling agents for microelectronic devices.
Literature: G. Boden et al. Keramische Zeitschrift 2005, 57(4), 220-223 and 2005, 57(5), 304-307, R. Müller et al. Langmuir 2004, 20, 2598-2606, R. Müller et al. Journal of Adhesion 2000, 72, 65-83.
-->Publications
The following list is a selection of all publications of PD Dr. Rainer Müller You can find the full publication list under the Button "Pubilcations".
1. S. Stählke, A. Schneider, D.B. Nebe, R. Müller. Response of osteoblast-like cells to polyamidoamine dendrimer-based surface coatings exhibiting different terminal functional groups, Materials Science & Engineering C, 2019, 101, 190-203.
2. S. Liu, B. Sandner, L.S. Nicholson, T. Schackel, L. Tenenbaum, R. Puttagunta, R. Müller, N. Weidner, A. Blesch. Regulated viral brain-derived neurotrophic factor delivery in combination with Schwann cells promotes axonal regeneration through alginate capillary hydrogels after spinal cord injury, Acta Biomaterialia, 2017, 60, 167-180 Available online at: http://epub.uni-regensburg.de/36191
3. M. I. Günther, N. Weidner, R. Müller, A. Blesch. Cell-seeded alginate hydrogel scaffolds promote directed linear axonal regeneration in the injured rat spinal cord. Acta Biomaterialia 2015, 27, 140-150.
Available online at: http://epub.uni-regensburg.de/36193
4. V. Katzur, M. Eichler, E. Deigele, C. Stage, P. Karagerogiev, J. Geis-Gerstorfer, G. Schmalz, S. Ruhl, F. Rupp, R. Müller. Surface-immobilized PAMAM-dendrimers modified with cationic or anionic terminal functions: physicochemical surface properties and conformational changes after application of liquid interface stress. Journal of Colloid and Interface Science 2012, 366, 179-190. Available online at: http://epub.uni-regensburg.de/24992
5. M. Eichler, V. Katzur, L. Scheideler, M. Haupt, J. Geis-Gerstorfer, G. Schmalz, S. Ruhl, R. Müller, F. Rupp. The impact of dendrimer-grafted modifications to model silicon surfaces on protein adsorption and bacterial adhesion. Biomaterials 2011, 32, 9168-9179. Available online at: http://epub.uni-regensburg.de/24993
6. R. Müller, A. Eidt, K.-A. Hiller, V. Katzur, S. Imazato, S. Ruhl, H. Schweikl, G. Schmalz. Influences of protein films on antimicrobial or bacteria-repellent surface coatings in a model system, using silicon wafers, Biomaterials 2009, 30, 4921-4929. Available online at: http://epub.uni-regensburg.de/24932
7. P. Prang, R. Müller, A.A. Eljaouhari, K. Heckmann, W. Kunz, T. Weber, C. Faber, M. Vroemen, U. Bogdahn, N. Weidner. The promotion of oriented axonal regrowth in the injured spinal cord by alginate-based anisotropic capillary hydrogels. Biomaterials 2006, 27, 3560-3569. Available online at: http://epub.uni-regensburg.de/1038
8. R. Müller, J. Abke, E. Schnell, D. Scharnweber, R. Kujat, C. Englert, D. Taheri, M. Nerlich, P. Angele. Influence of surface pretreatment of titanium- and cobalt-based biomaterials on covalent immobilization of fibrillar collagen. Biomaterials 2006, 27, 4059-4068. Available online at: http://epub.uni-regensburg.de/24954
9. R. Müller, J. Abke, F. Macionczyk, U. Gbureck, Z. Ruszczak, R. Mehrl, R. Kujat, C. Englert, M. Nerlich, P. Angele. Surface engineering of stainless steel materials by covalent collagen immobilization to improve implant biocompatibility. Biomaterials 2005, 26, 6962-6972. Available online at: http://epub.uni-regensburg.de/24962
10. P. Angele, J. Abke, R. Kujat, H. Faltermeier, D. Schumann, M. Nerlich, B. Kinner, C. Englert, Z. Ruszczak, R. Mehrl, R. Müller. Influence of different collagen species on physico-chemical properties of crosslinked collagen matrices. Biomaterials 2004, 25, 2831-2841. Available online at: http://epub.uni-regensburg.de/24967
open Positions
No theme in the moment!!!


