
Research Area A
| Project Area A: (Patho-) physiology of the tubular system |
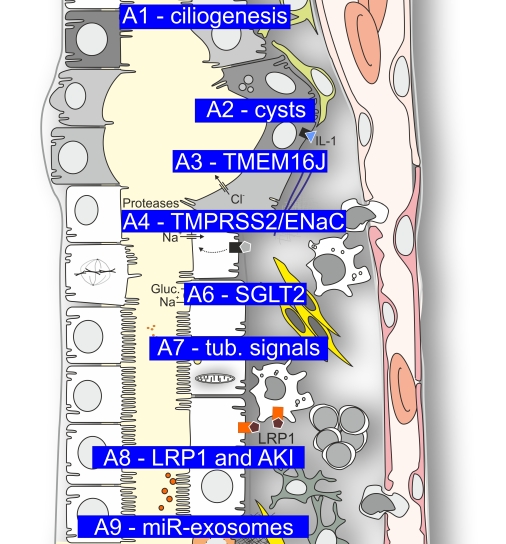
Mechanisms of early ciliogenesis
Prof. Dr. Ralph Witzgall
Molecular and Cellular Anatomy
Universität Regensburg
Primary cilia have limited function in an ever increasing number of illnesses, now also known as ciliopathies. The underlying pathogenetic processes can only be understood if the formation of primary cilia is extensively characterized. According to findings made during the ongoing funding period endocytosis and the Golgi apparatus are important for ciliogenesis. In the current proposal we want to investigate in what way endocytotic processes contribute to the formation of cilia, when the Golgi apparatus is involved, which proteins are active at the Golgi apparatus and which proteins facilitate the docking and the fusion of transport vesicles at the mother centriole.
The impact of ROS, ferroptosis, glucose transport and IL-1 receptor signaling on the progression of ADPKD
PD Dr. Björn Buchholz
Nephrology
Friedrich-Alexander-Universität Erlangen-Nürnberg
In the previous funding period, we identified calcium-activated chloride secretion induced by HIF-1α and mediated by the chloride channel Anoctamin 1 as a significant contributor to cyst growth in polycystic kidney disease. In this funding period, we will address the impact of HIF-induced but secretion-independent signaling pathways on cyst progression in vitro and in vivo by inhibition of GLUT1-mediated glucose transport, use of approved HIF-PHD inhibitors, antagonism of IL-1 receptor signaling, application of the antioxidant coenzyme Q10, and inhibition of ferroptosis.
TMEM16J in chronic kidney disease
Prof. Dr. Rainer Schreiber
Physiology
Universität Regensburg
Prof. Dr. Karl Kunzelmann
Physiology
Universität Regensburg
During the present funding period, a genome wide association study (project C6) demonstrated association of the proteins TMEM16J and SIGIRR with chronic kidney disease (CKD). Earlier work from our team demonstrated TMEM16J as an intracellular protein that inhibits intracellular Ca2+ signaling, which affects numerous cellular functions such as volume regulation, cell death and proliferation. TMEM16J, with the help of SIGIRR and PKP3, also controls inflammation in various tissues. We found that TMEM16J is expressed in the kidney and will determine its renal function and role in CKD. To this end, we study expression, function and the role of TMEM16J in mouse models for CKD, using TMEM16J knockout and knockin mice, and kidneys obtained from CKD patients.
Tubular and interstitial proteases as regulators of the epithelial sodium channel (ENaC)
Prof. Dr. Christoph Korbmacher
Physiology
Friedrich-Alexander-Universität Erlangen-Nürnberg
Dr. Alexandr Ilyaskin
Physiology
Friedrich-Alexander-Universität Erlangen-Nürnberg
Recently, we identified TMPRSS2 as novel candidate protease to activate the epithelial sodium channel (ENaC) by proteolytic cleavage. We aim to elucidate the role of TMPRSS2 in renal ENaC regulation and gain further insights into the complex molecular mechanisms of proteolytic and non-proteolytic ENaC activation. A better understanding of ENaC function at the molecular level may lead to novel (patho-) physiological and therapeutic concepts for renal disorders involving increased ENaC activity and renal salt retention.
Cardio- and nephroprotective mechanisms of SGLT2 inhibition
Prof. Dr. Lars Maier
Cardiology
Universität Regensburg
Prof. Dr. Stefan Wagner
Cardiology
Universität Regensburg
SGLT2 inhibitors (SGLT2i) have substantial beneficial effects on renal and cardiac function but underlying mechanisms are unknown. We will investigate the role of cardiac Na+ channels for SGLT2i-dependent inhibition of cardiac injury, the impact of SGLT2i-improved ejection fraction for renal protection in mice with heart failure induced by transverse aortic constriction. Furthermore, we will identify molecular mechanisms of SGLT2i-induced microalbuminuria and renal myo-inositol loss and explore the consequences of these pathways for cardiac function in mice and patients with heart failure. The results will shed light on new mediators and mechanisms of cardio-renal crosstalk upon SGLT2i.
Proximal tubular signals as determinants of renal inflammation and fibrosis
Prof. Dr. Richard Warth
Medical Cell Biology
Universität Regensburg
Prof. Dr. Christine Ziegler
Structural Biology
Universität Regensburg
In recent years, we have investigated the pathophysiology of GATM- and EHD1-related monogenetic diseases involving proximal tubular mitochondria and intracellular vesicle dynamics, respectively. Here, we will investigate the role of EHD1 in intracellular membrane shaping and dynamics and the adaptive mechanisms underlying the mild phenotype of GATM knockin mice. The expected results will help to elucidate the functional principles of proximal tubule adaptation and maladaptation and ideally lead to the development of a therapeutic approach for GATM-associated Fanconi syndrome.
The role of Lipoprotein Receptor-Related Protein 1 (LRP1) in acute kidney injury
Dr. Tilman Jobst-Schwan
Medizinische Klinik 4 - Nephrologie und Hypertensiologie
Universitätsklinikum Erlangen
Prof. Dr. Mario Schiffer
Medizinische Klinik 4 - Nephrologie und Hypertensiologie
Universitätsklinikum Erlangen
LRP1 is an ubiquitously expressed membrane receptor that is involved in Wnt/β-catenin signaling and in cholesterol metabolism but also in modulation of immune cells. These mechanisms are all involved in the pathogenesis of acute kidney injury (AKI). Our preliminary experiments suggest LRP1 agonism as a plausible nephroprotective therapeutic approach corresponding to published data in myocardial infarct treatment. Thus, we will investigate the role of LRP1 in AKI as an experimental fundament for a clinical study.
Resolving glomerular to tubular crosstalk through miR-containing exosomes
Prof. Dr. Janina Müller-Deile
Medizinische Klinik 4 - Nephrologie und Hypertensiologie
Universitätsklinikum Erlangen
Prof. Dr. Stefan Uderhardt
Medizinische Klinik 3 - Rheumatologie und Immunologie
Universitätsklinikum Erlangen
The mechanisms that cause tubular involvement in glomerular diseases are still unclear. We hypothesize that previously unrecognized intercompartmental communication networks exist between glomerular and tubular cells via miRNA (miR)-loaded exosomes. This will be investigated using cell cocultures and transgenic zebrafish lines, which will allow us to dynamically track exosomes in vitro and in vivo and determine the effects on autophagy, inflammatory cascades as well as tissue recovery after tubular injury. As a translational approach, miR-containing exosomes from the urine of patients with glomerular diseases will be investigated as potential non-invasive biomarkers of tubular injury.
MISSION
"Interdisciplinary kidney research to advance understanding of disease mechanisms and develop new therapeutic concepts"
Contact:
Dr. Michaela Kritzenberger
Email
Tel.: ++49 (0)941/943-2885

